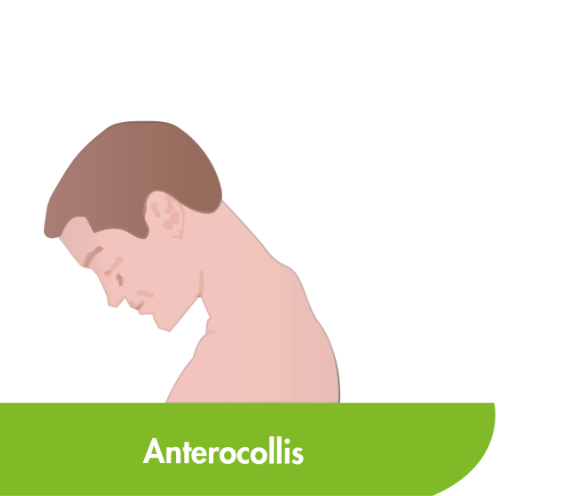
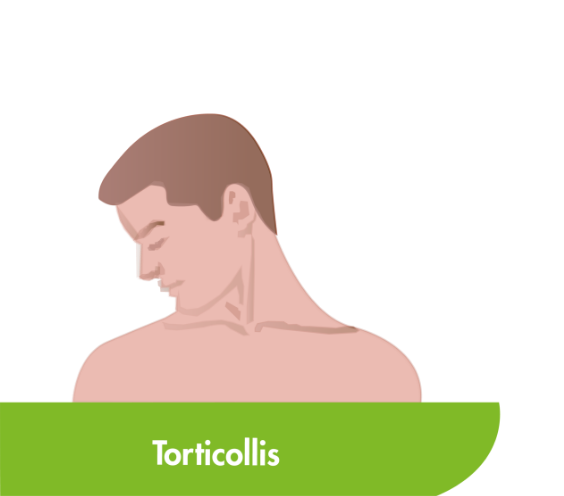
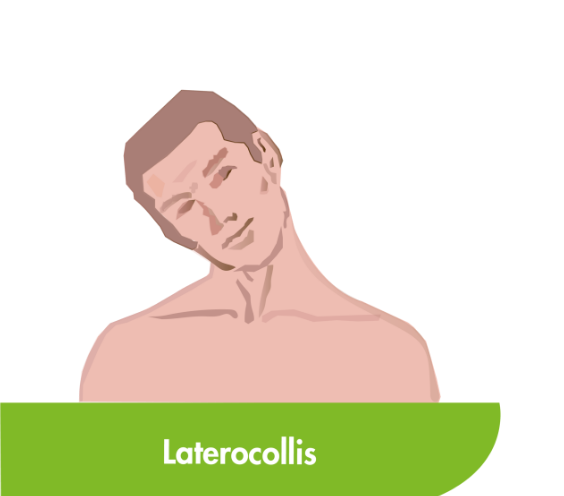
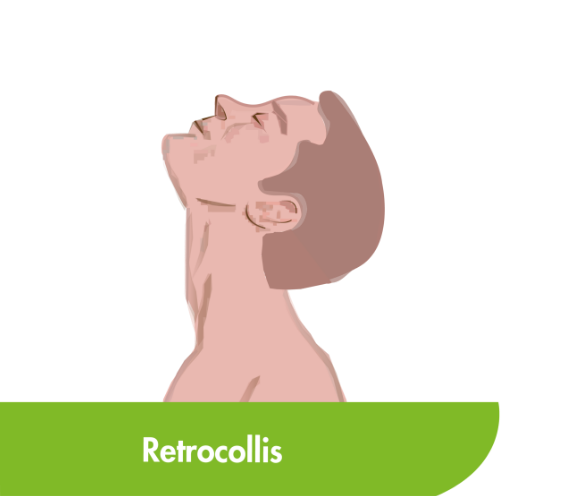
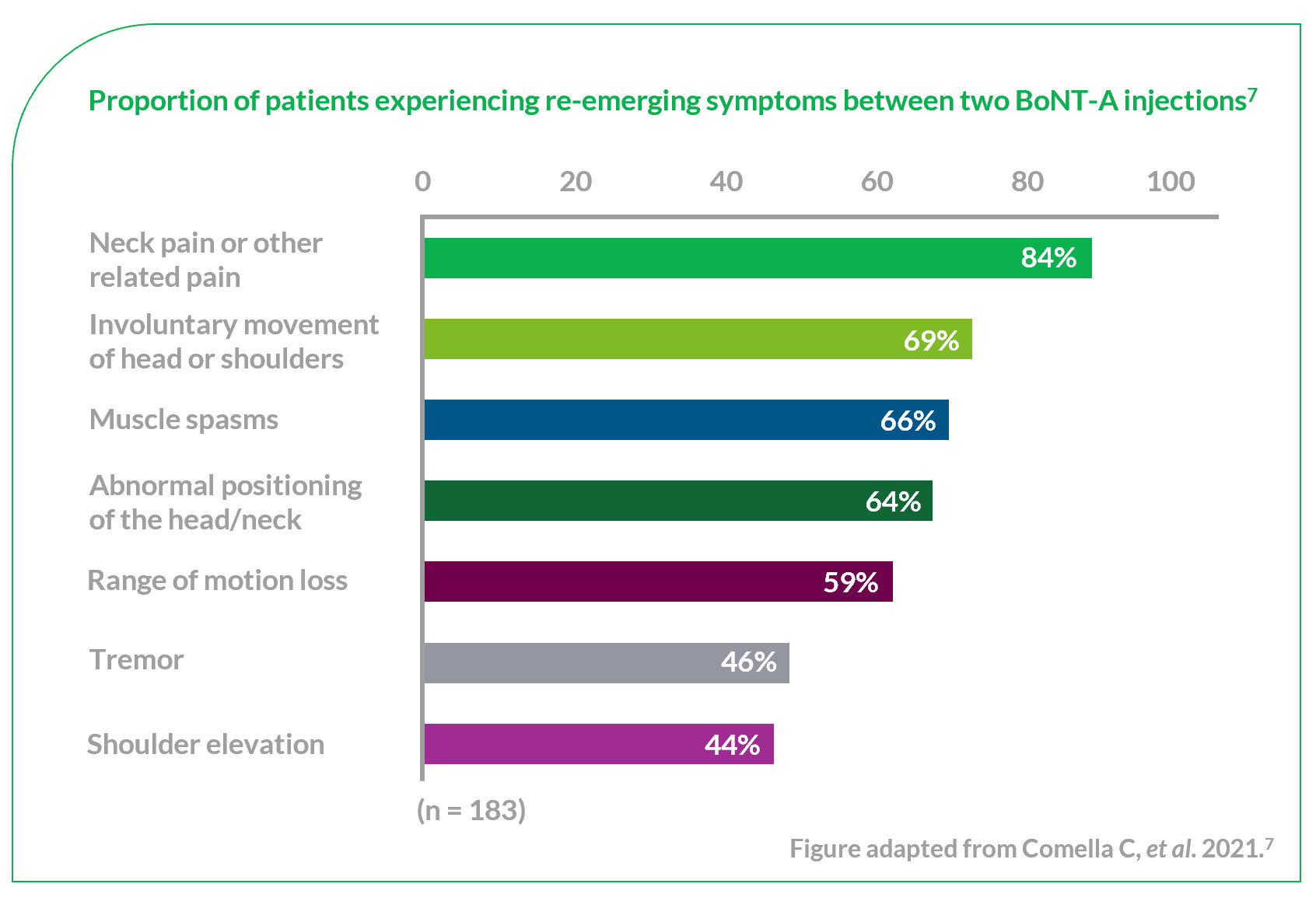
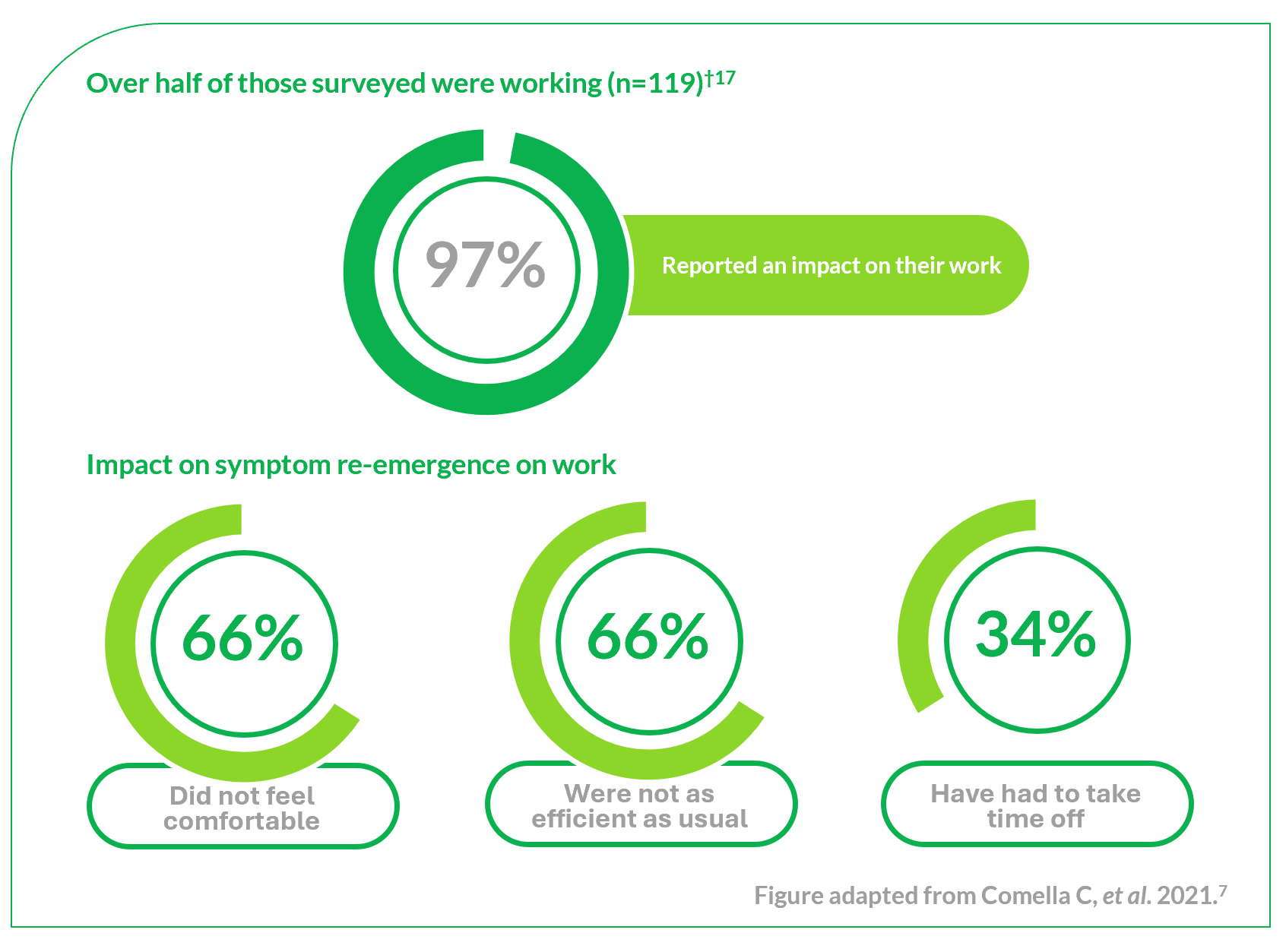
CD is the most common type of focal dystonia encountered in neurological practice.1
In patients with CD, painful and involuntary patterned postures of intermittent spasms of the neck muscles cause the head to rotate, tilt or move away from its normal position or posture.2,3
To help select the correct muscles for treatment, subtypes of CD have been proposed based on the anatomic organisation and action of dystonia of the main cervical muscles.3,4 Torticollis, the most common form of CD, can induce rotation in the head but primarily involves muscles at the C2-C7 level.2–4