This webpage is designed to be viewed on a laptop screen or larger.
Dysport® injections may be repeated approximately every 16 weeks, or as required to maintain a response, but not more frequently than every 12 weeks.1
This website has been commissioned by Ipsen Pharmaceuticals Ltd. and is intended for Irish Healthcare Professionals. If you are not a Healthcare Professional, please click here.
Adverse event reporting information is available at the bottom of this webpage.
This website has been commissioned by Ipsen Pharmaceuticals Ltd. and is intended for an Irish audience

This webpage is designed to be viewed on a laptop screen or larger.
Dysport® injections may be repeated approximately every 16 weeks, or as required to maintain a response, but not more frequently than every 12 weeks.1
Mean time to retreatment over repeated cycles (n=108)15(±5.6)-17(±8.0) WEEKS OVER THREE OPEN-LABEL CYCLES |
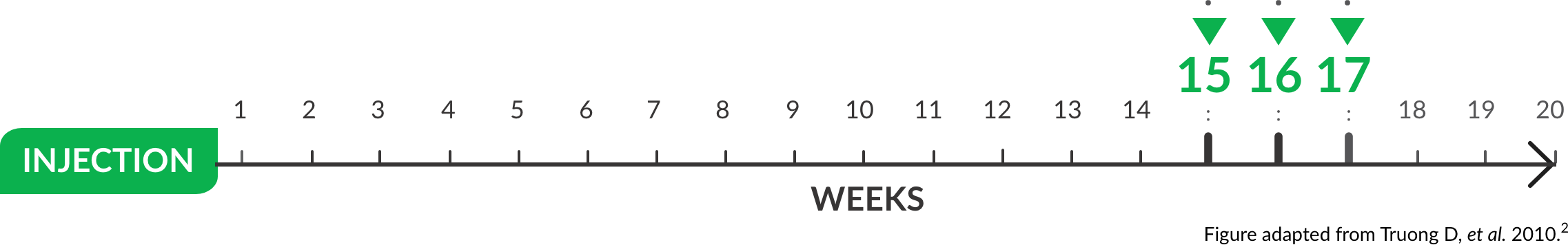
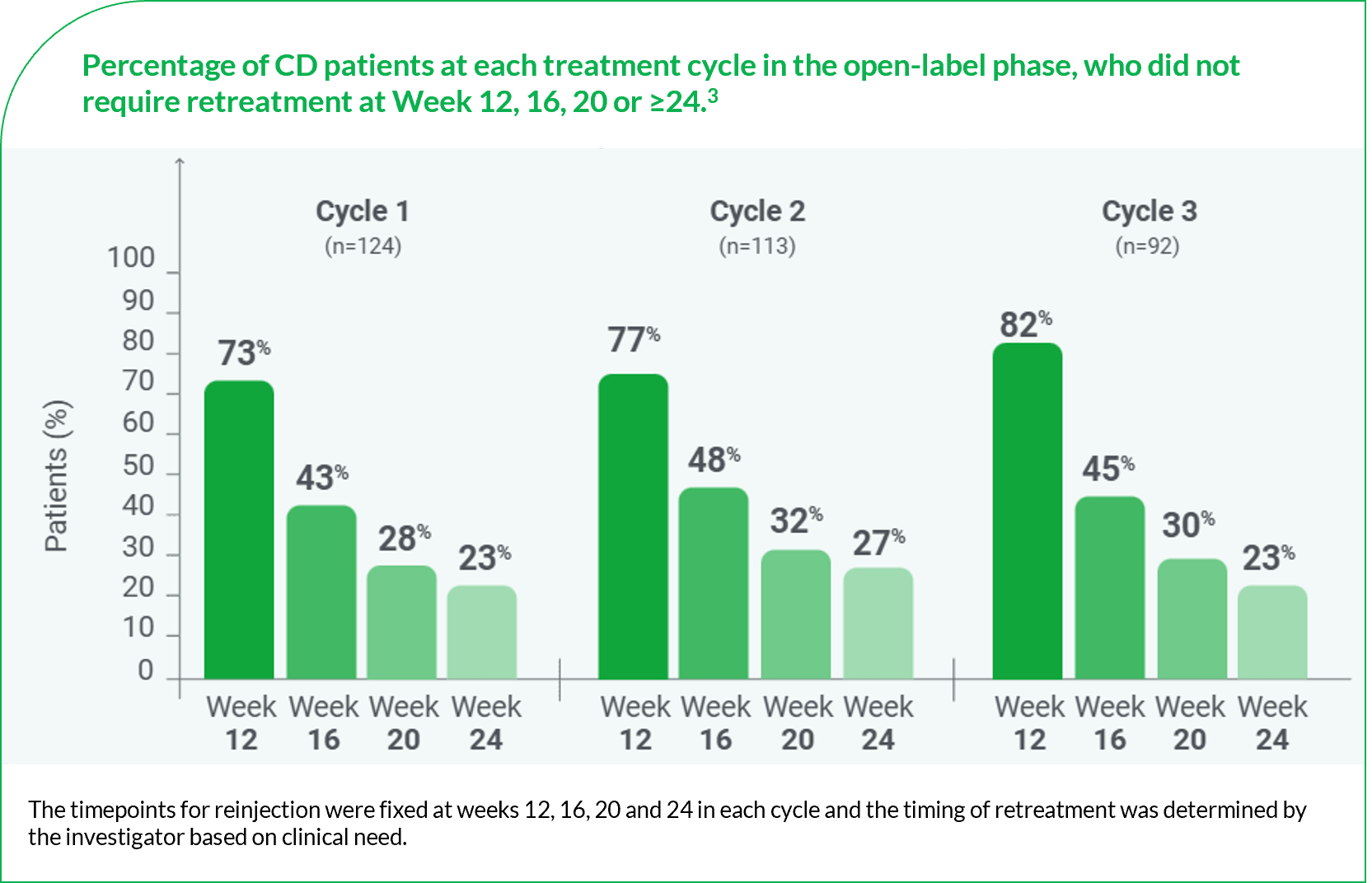
Figure adapted from Esquenazi A, et al. 2020.3
| Indication | Level of recommendation‡ | |||
|---|---|---|---|---|
| Level A | Level B | Level C | Level U | |
| Cervical dystonia | Dysport® | OnabotulinumtoxinA IncobotulinumtoxinA |
Table adapted from AAN Guidelines.6
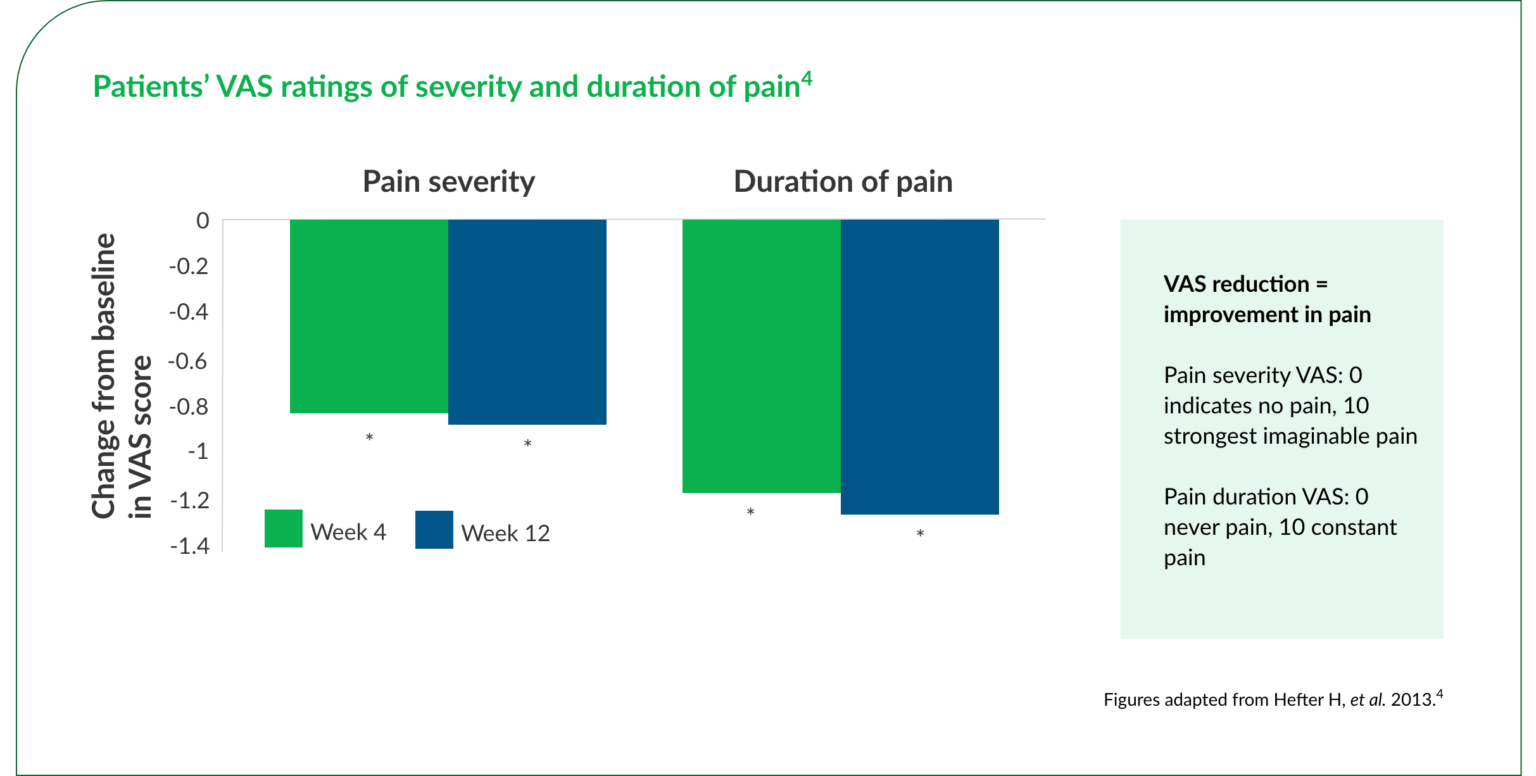
*Statistically significant (P<0.001) vs baseline.
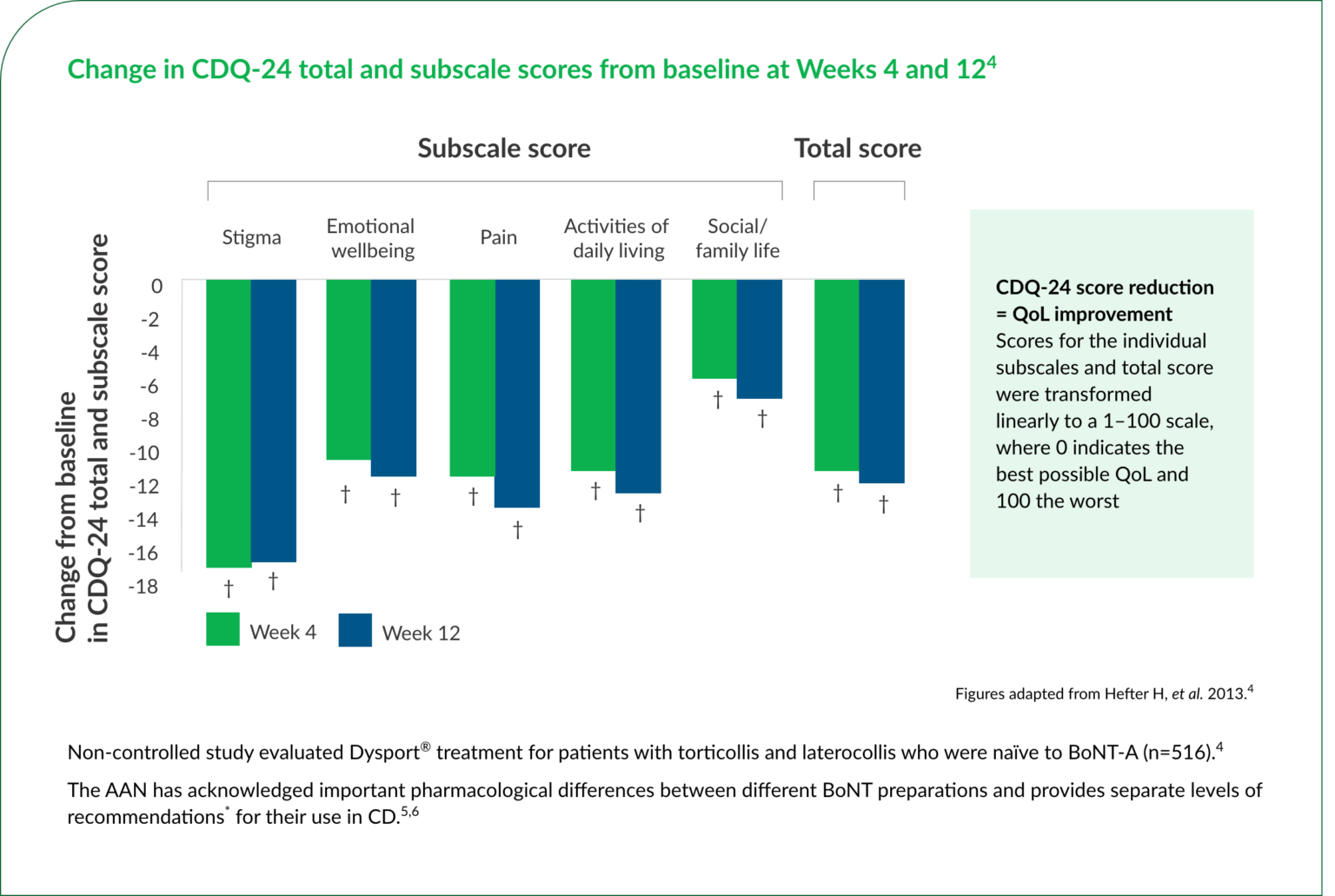
†Statistically significant (P<0.001) vs baseline change from baseline score was worse at weeks 12 to 4.
‡Level A: established as effective, or useful; should be offered.6 Level B: probably effective, or probably useful; should be considered.6 Level C: possibly effective, or possibly useful; may be considered.6 Level U: data inadequate or conflicting; insufficient to support or refute a benefit.6
AAN, American Academy of Neurology; BoNT-A, BoNT-A, botulinum toxin type A; CD, cervical dystonia; CDQ-24, Craniocervical Dystonia Questionnaire; QoL, quality of life; VAS, visual analogue scale.
For further information, please refer to the Prescribing Information or the Summary of Product Characteristics.
Adverse events should be reported.
Reporting forms and information can be found at www.hpra.ie or e-mail medsafety@hpra.ie.
The HPRA can also be contacted on +353 16764971. Adverse events should also be reported to Ipsen via email at pharmacovigilance.uk-ie@ipsen.com or phone on +353 1 8098256.
Reporting of side effects:
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly to the HPRA. Reporting forms and information can be found at www.hpra.ie or email medsafety@hpra.ie. Adverse events should also be reported to Ipsen via email at pharmacovigilance.uk-ie@ipsen.com or phone on +353 1 8098256. By reporting side effects, you can help provide more information on the safety of this medicine.