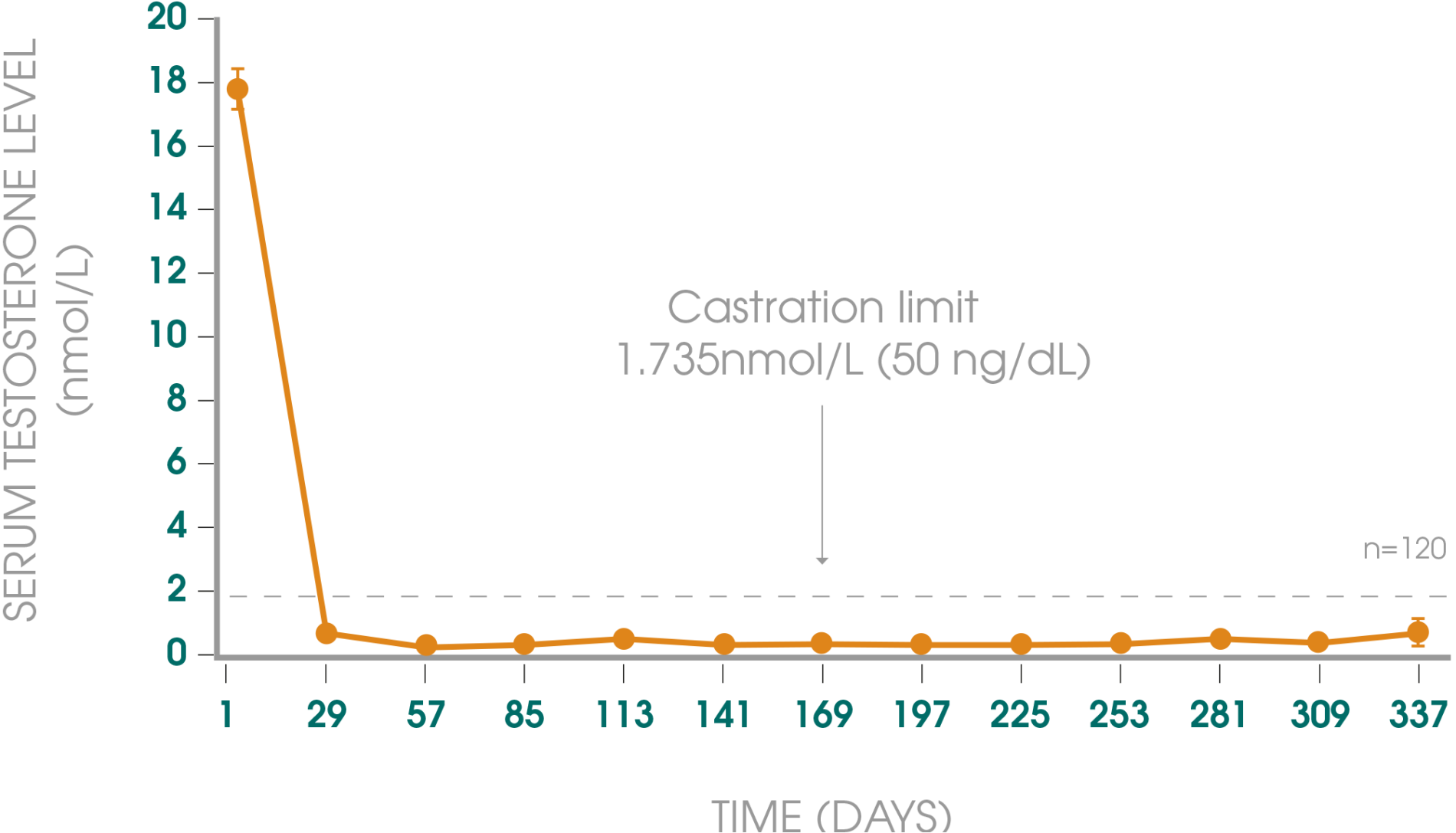
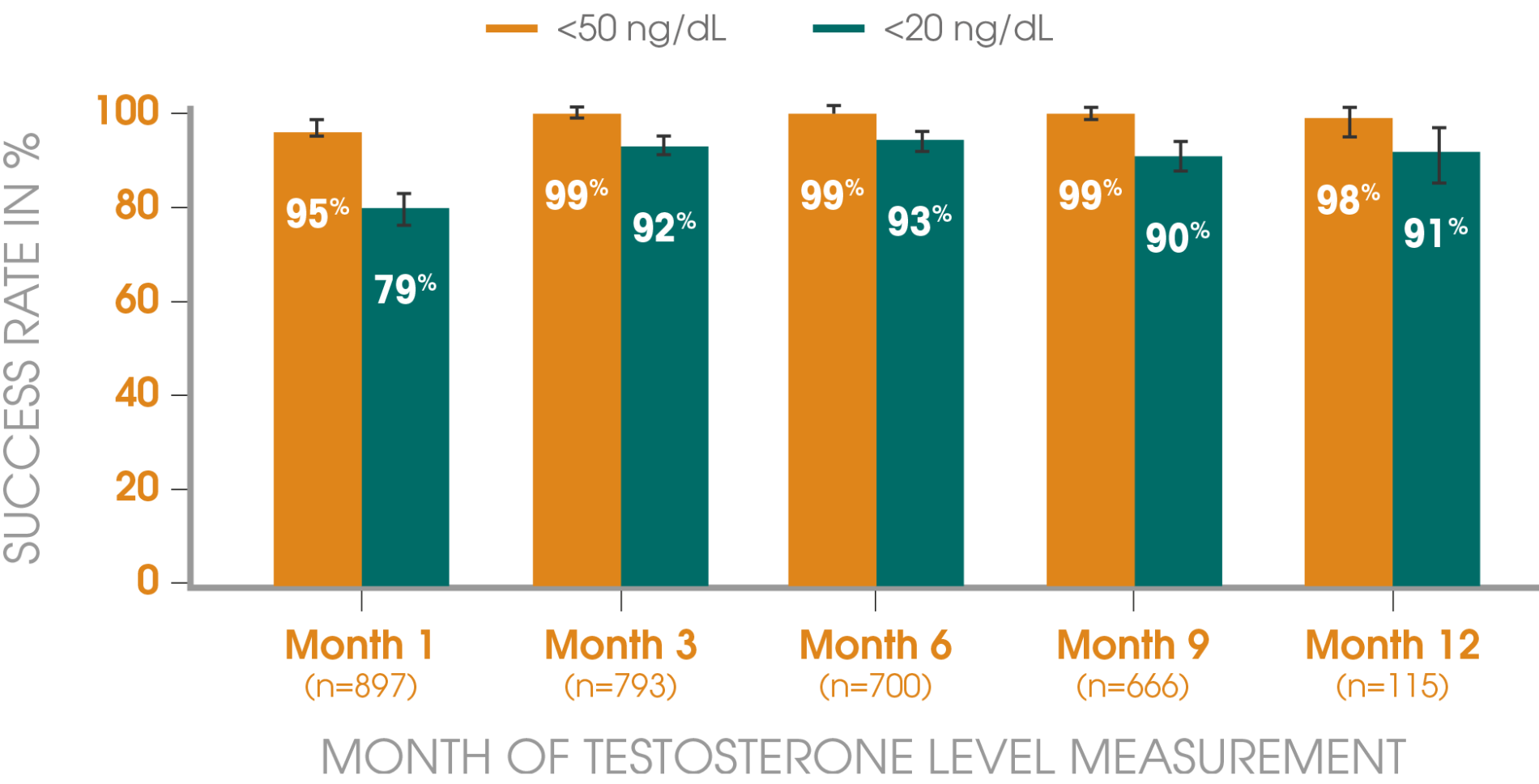
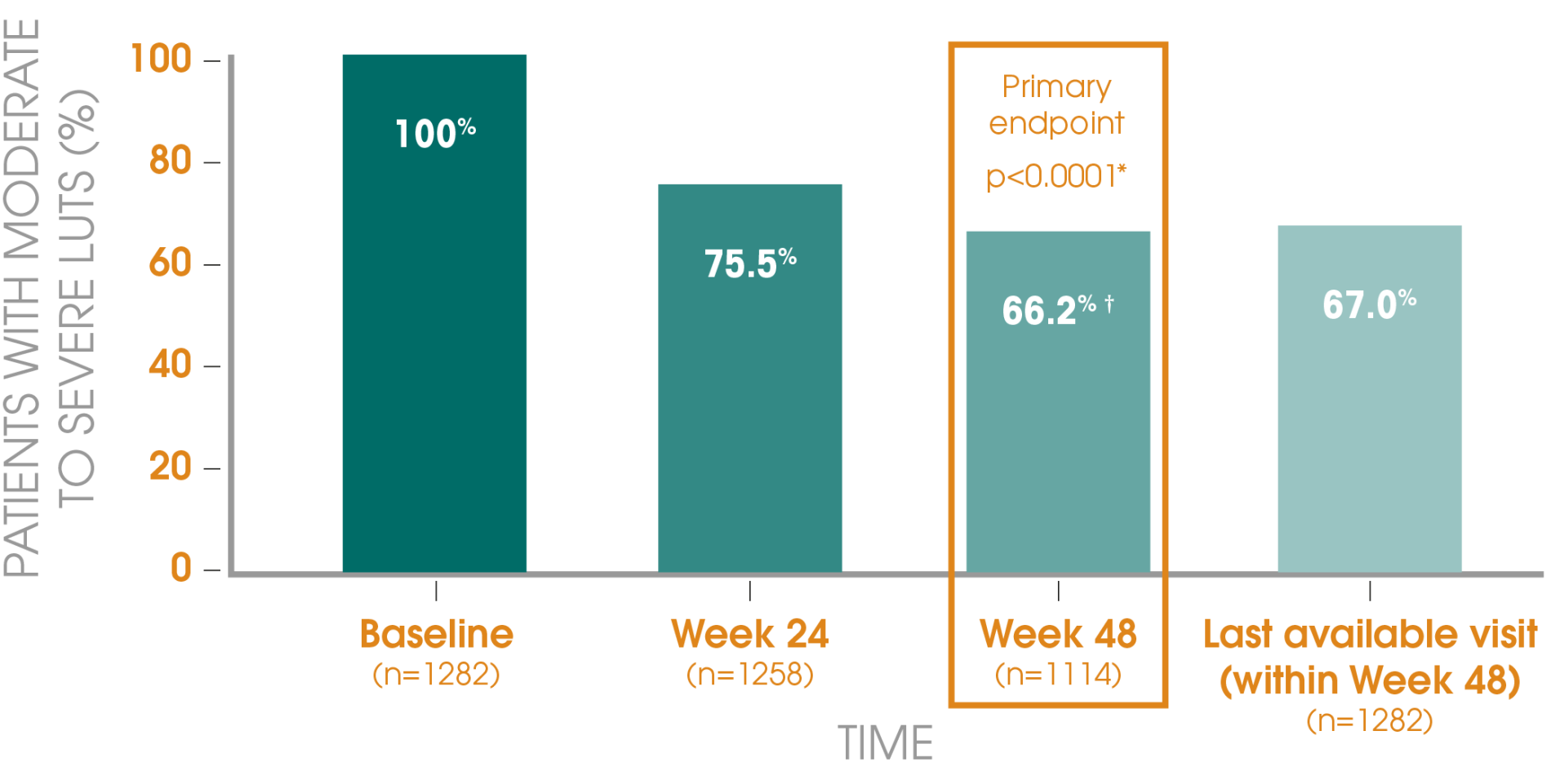
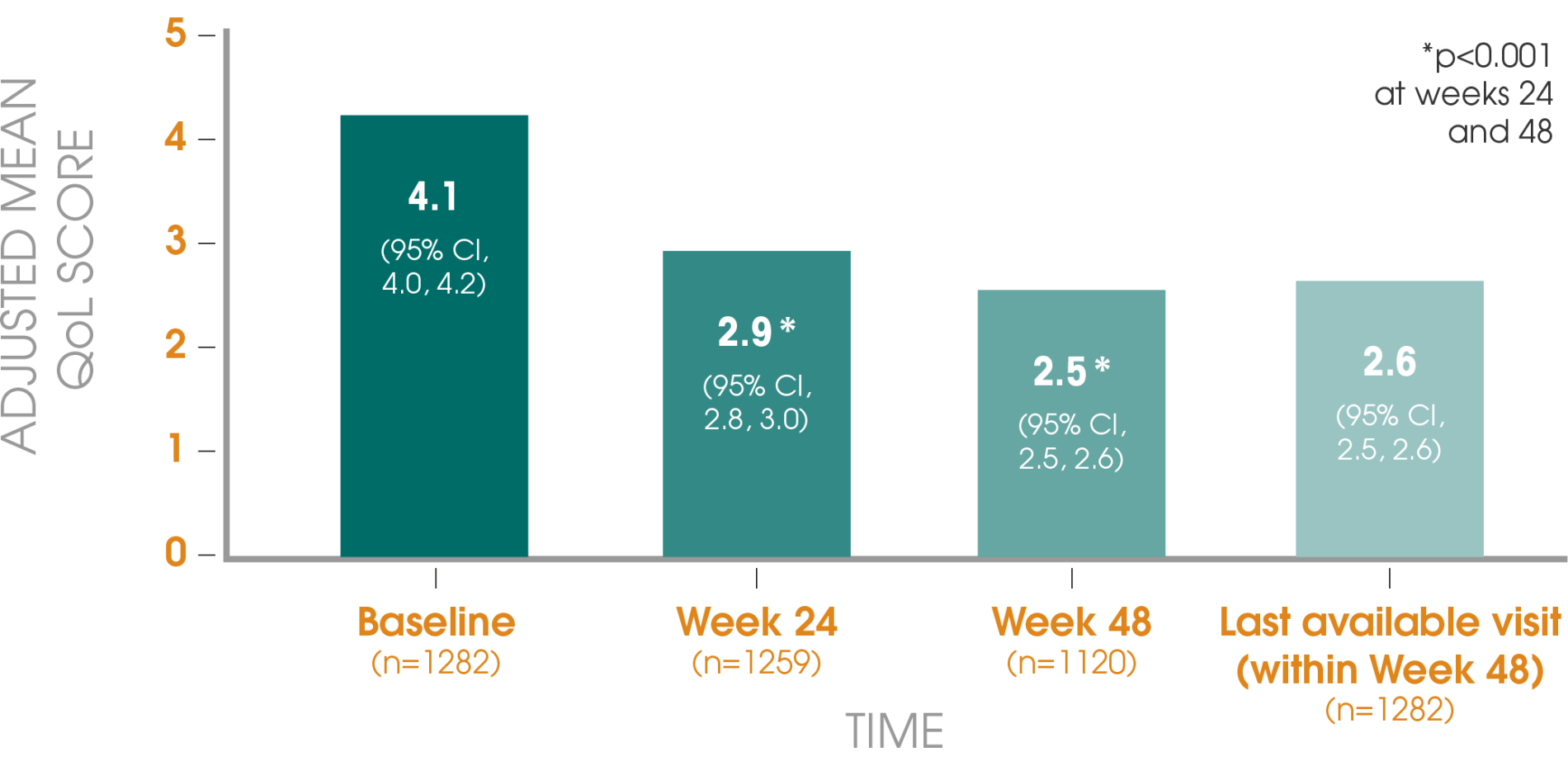
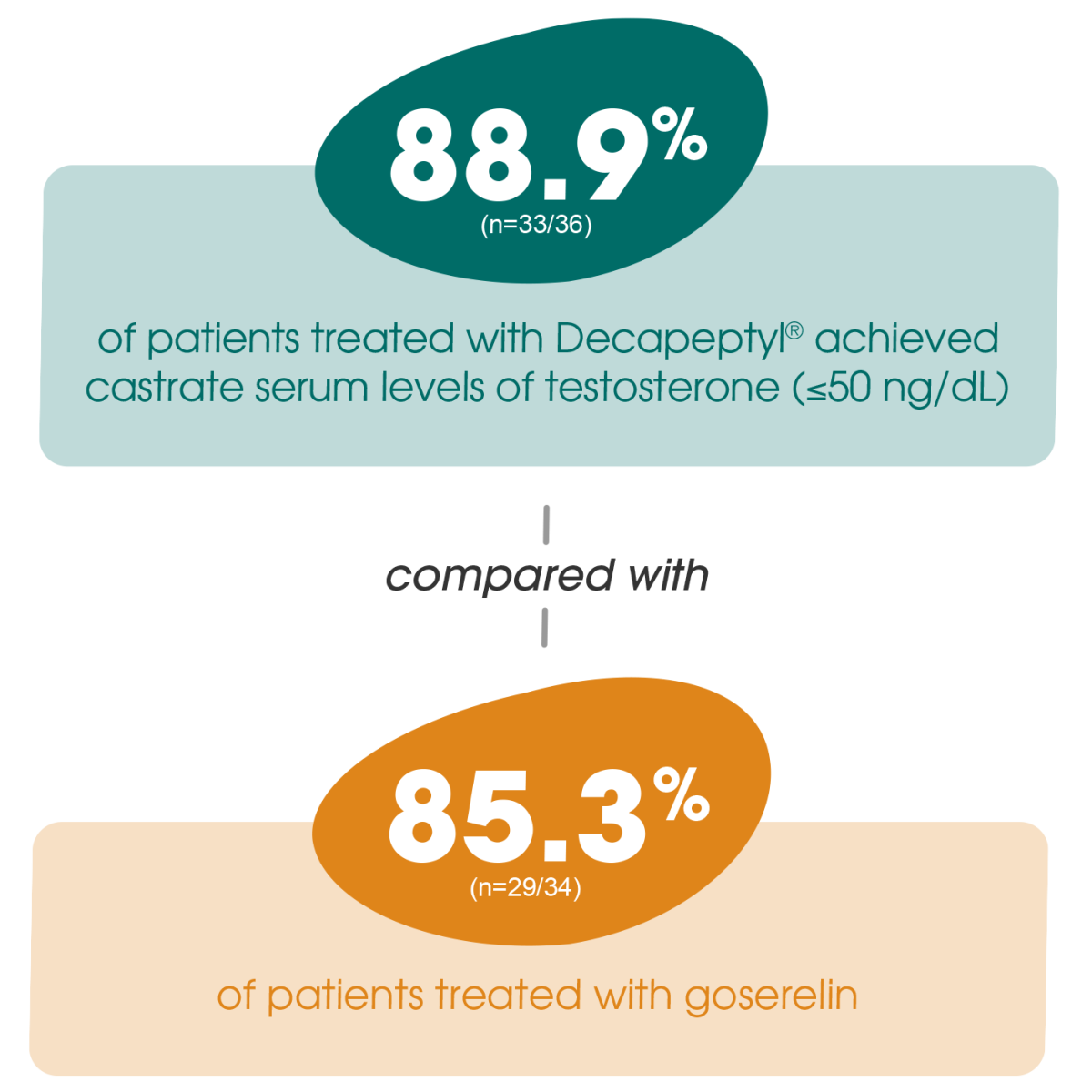
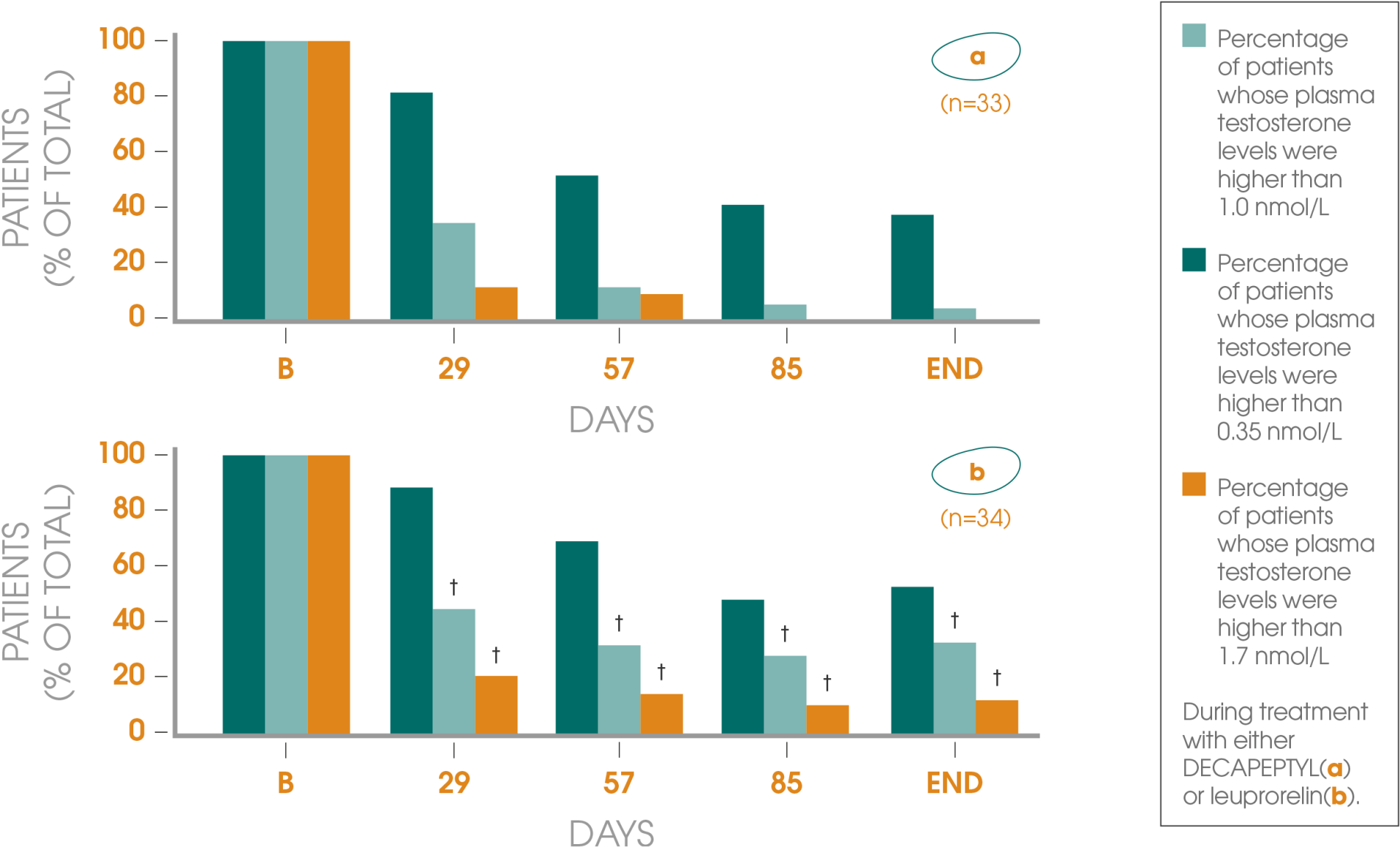
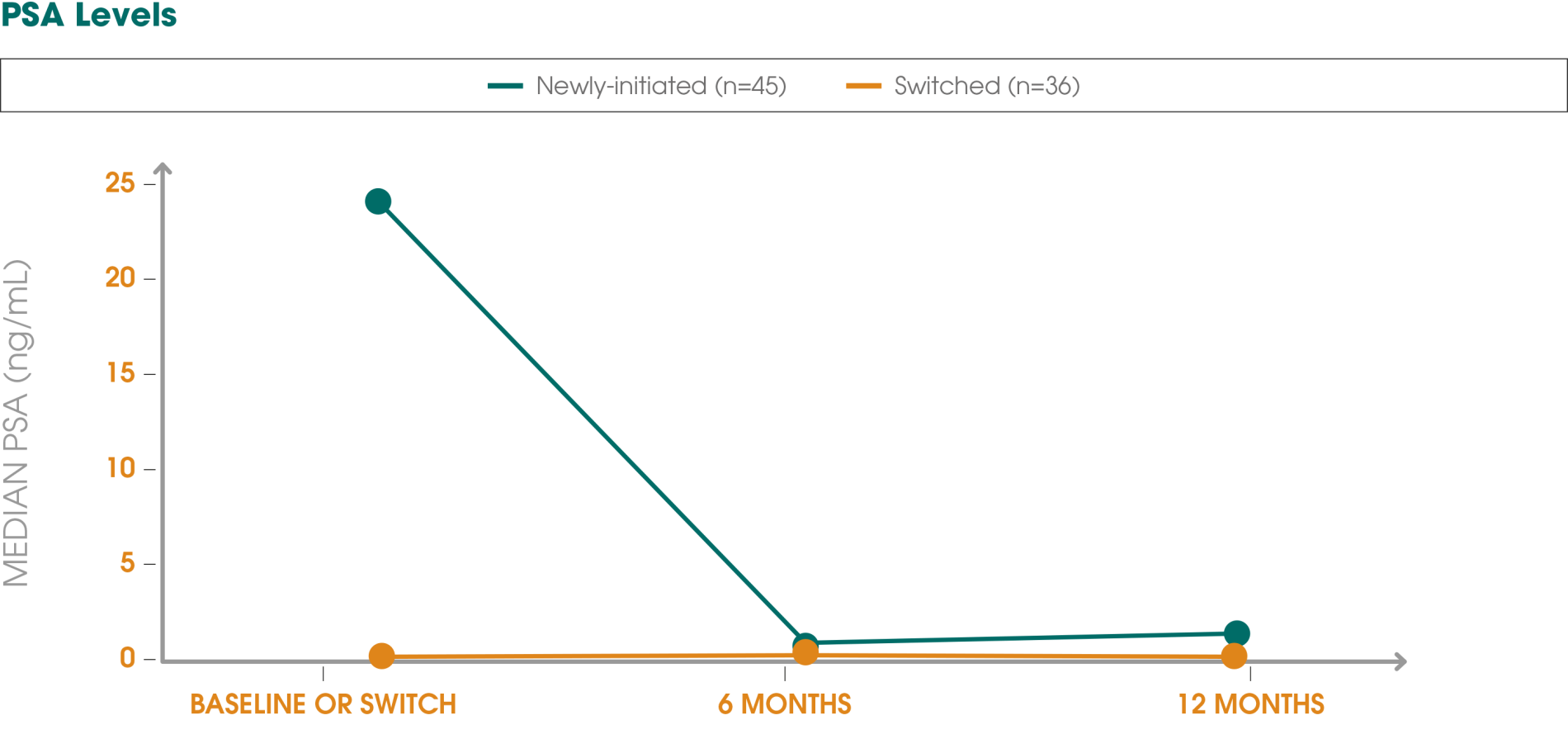
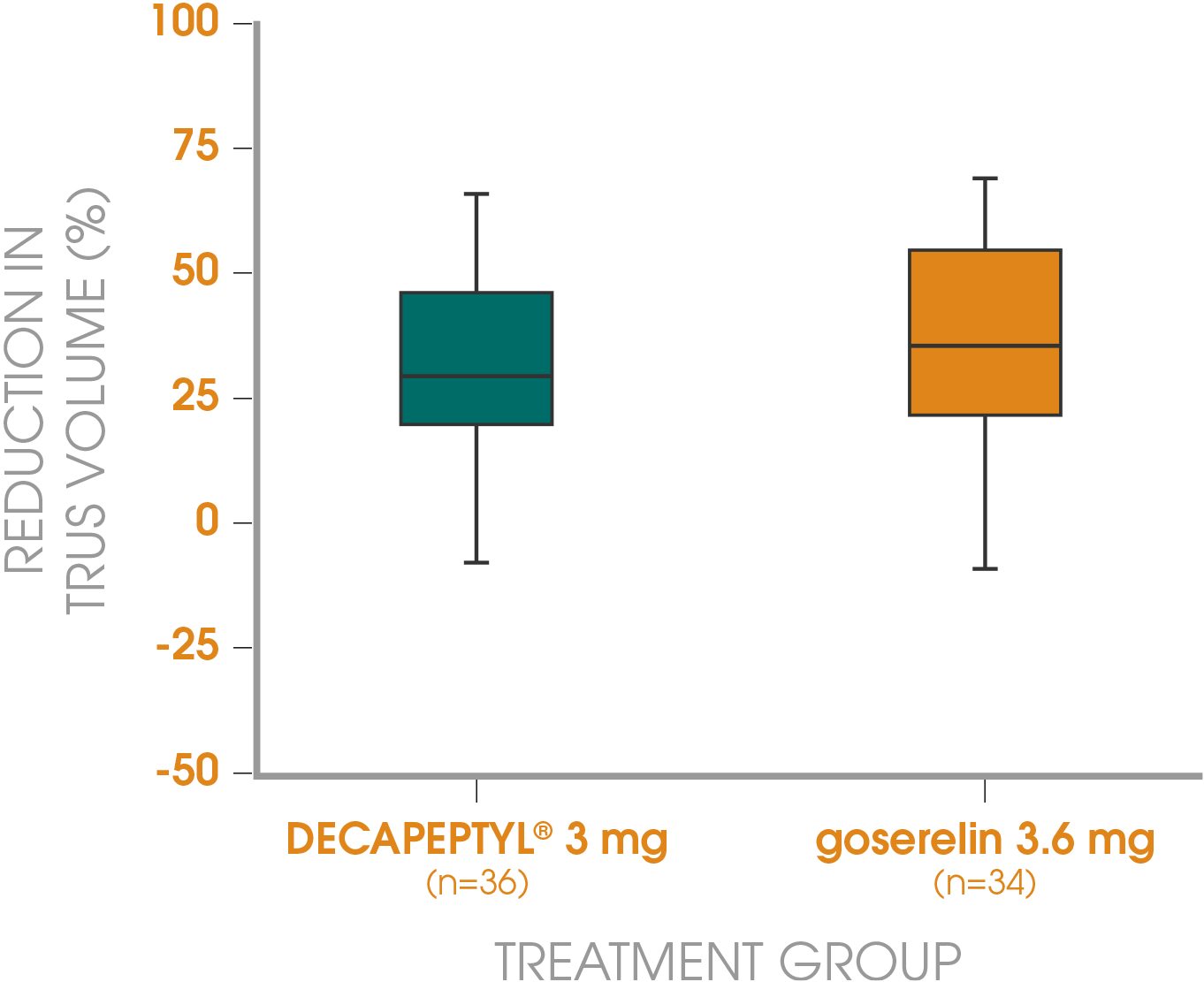Adapted from Gil T et al., 2015.3
*For overall time effect at week 48.
†According to total International Prostate Symptom Score (IPSS).
Study information3
Design: Prospective, non-interventional, multicentre studies of lower urinary tract symptoms (LUTS) conducted in Algeria, Belgium, China, Hungary, Romania and South Korea.
Aim: To evaluate the effect of Decapeptyl® on lower urinary tract symptoms (LUTS) in patients with advanced prostate cancer.
Primary endpoint: The primary efficacy endpoint was the proportion of patients with moderate or severe LUTS after 48 weeks, as assessed by the International Prostate Symptom Score (IPSS). Secondary endpoints included the distribution of IPSS categories, total IPSS and prostate-specific antigen (PSA) levels at baseline, 24 and 48 weeks.
Patients: In total, 2461 patients were recruited; 1282 had moderate or severe LUTS at baseline, as signified by an IPSS of greater than 7.
Treatment: Decapeptyl® 1- or 3-monthly.