Decapeptyl® contains triptorelin, which is a synthetic analogue of luteinising hormone-releasing hormone (LHRH), also known as gonadotrophin-releasing hormone (GnRH).1-3
Triptorelin acts as a potent inhibitor of gonadotropin secretion when given continuously and in therapeutic doses.1-3
After administration of triptorelin, there is an initial and transient increase in circulating levels of luteinising hormone (LH), follicle-stimulating hormone (FSH) and testosterone in males and oestradiol in females.1-3
Continuous and long-term administration of triptorelin, however, results in decreased LH and FSH secretion and suppression of testicular and ovarian steroidogenesis.1-3
Decapeptyl® leads to testosterone suppression in prostate cancer patients
| Decapeptyl® mechanism of action |
| Injection of Decapeptyl® |
Phase 1 |
↑↑ LH, testosterone |
Potential flare effect |
Phase 2
(starting from 2 weeks) |
↓↓ LH, testosterone |
Desensitisation phase |
Sustained release formulation
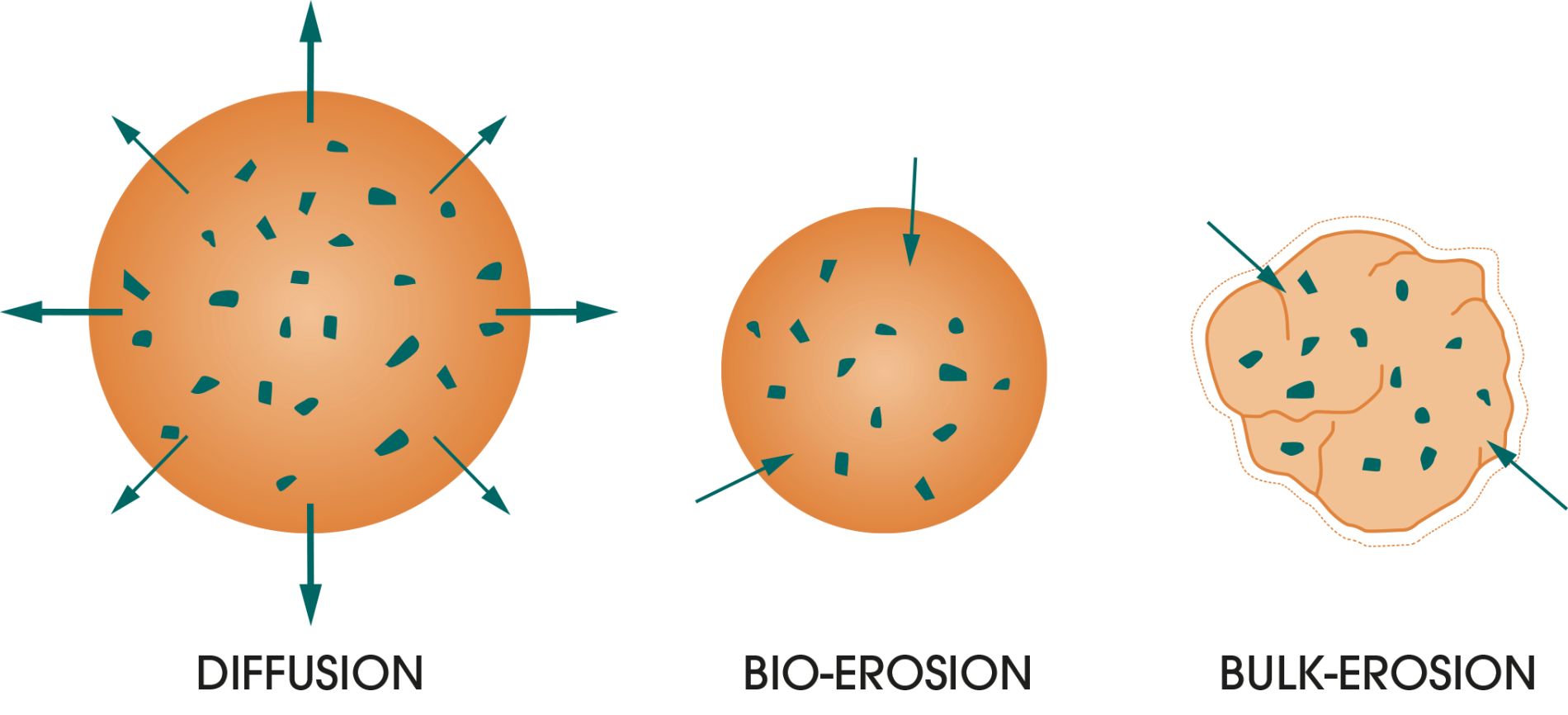
Triptorelin is contained within microspheres that are designed for sustained release.4
Microspheres are spherical particles that consist of a continuous network of support material or polymer in which the active substance is dispersed.
The different stages of triptorelin microsphere breakdown4