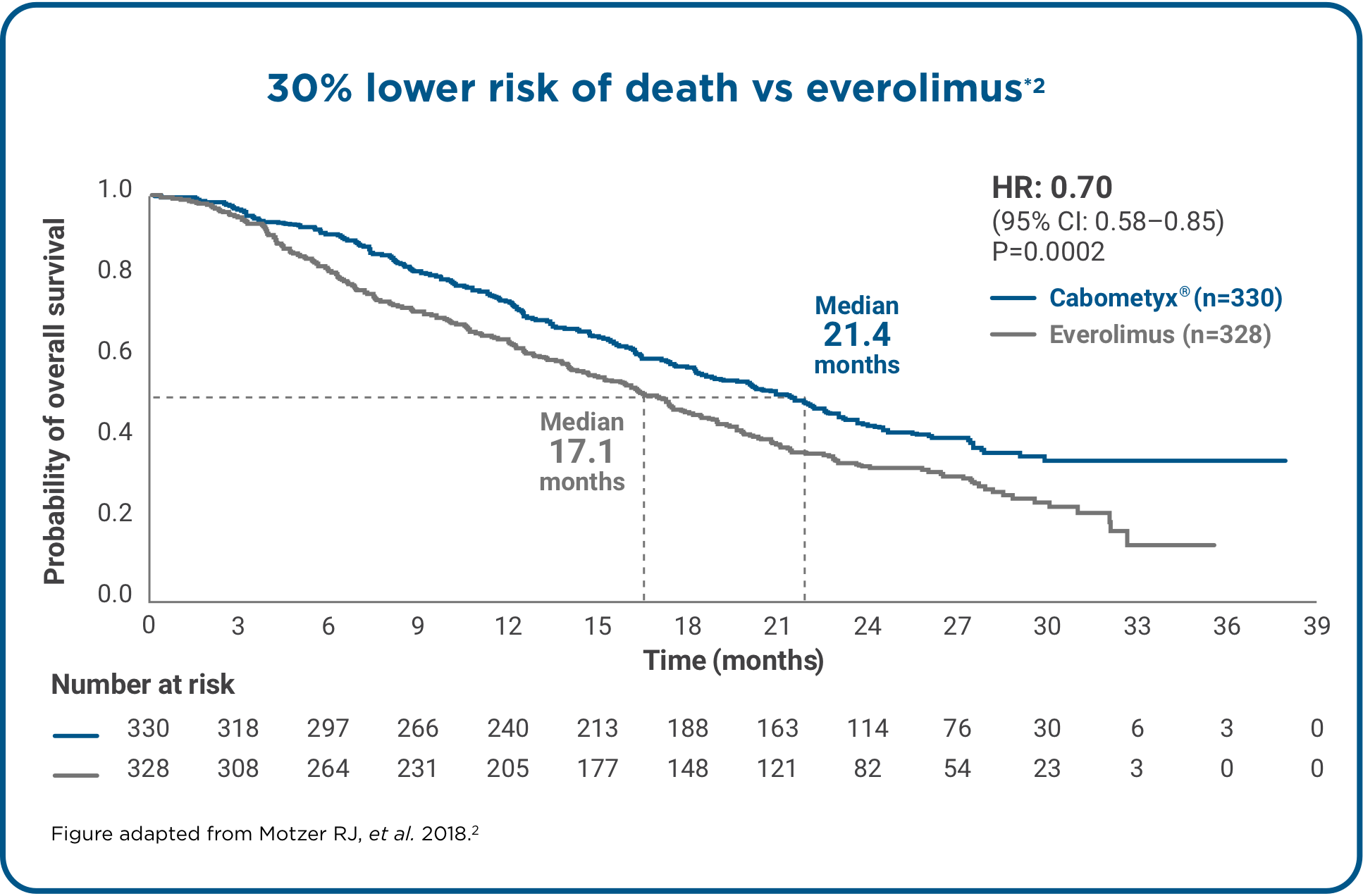
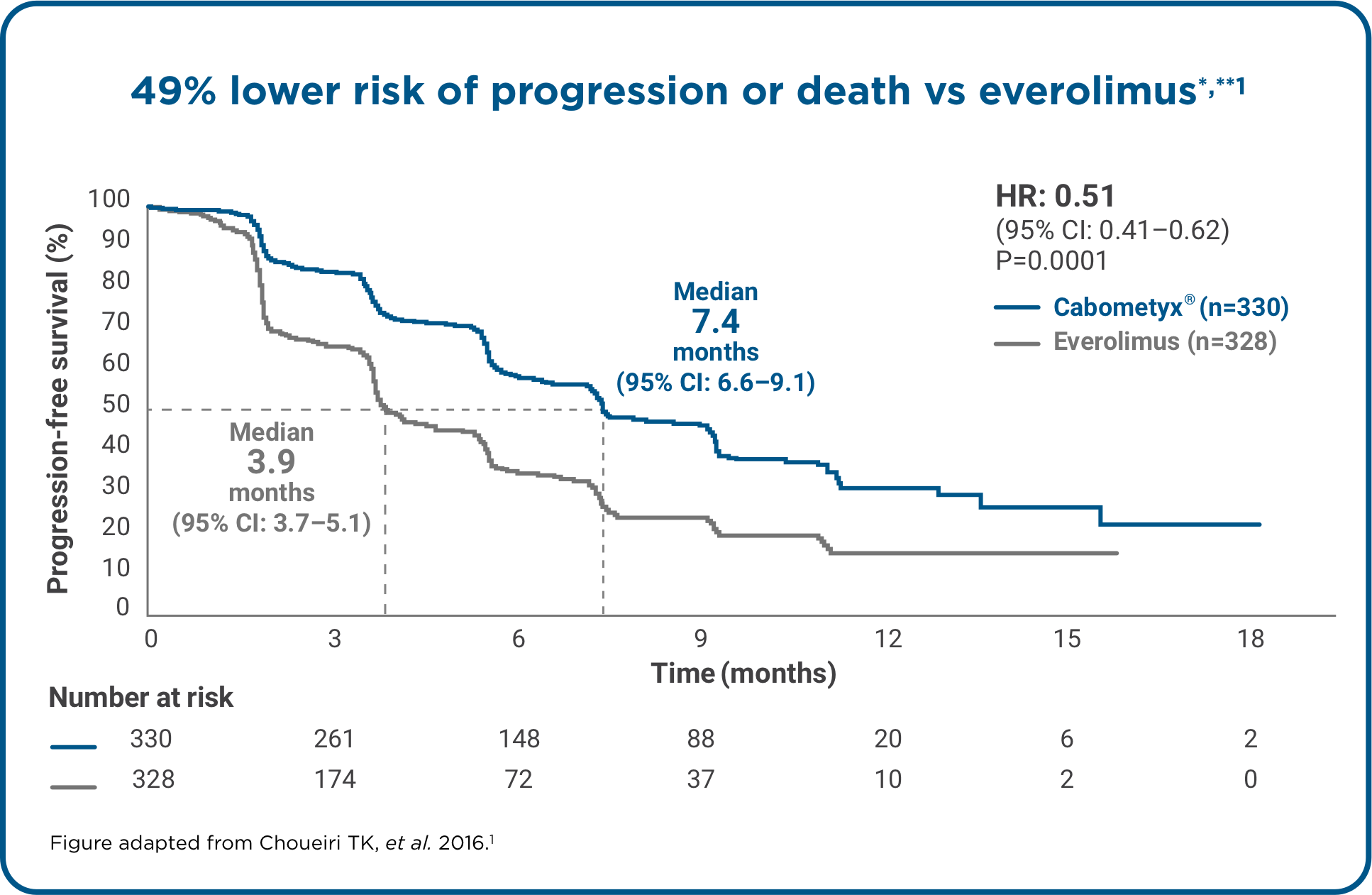
Cabometyx® is the only TKI to show longer overall survival, superior PFS and ORR vs everolimus.*1,2

Efficacy Information
Footnotes
*Data from METEOR, a Phase 3, randomised, open-label study comparing Cabometyx® monotherapy (n=330) with everolimus (n=328) in adult patients with aRCC progressing after prior anti-VEGF therapy. PFS was the primary endpoint (assessed by IRC in the first 375 patients who underwent randomisation) and ORR and overall survival were secondary endpoints of the study.1,2 Median overall survival data cut-off: October 2016. Median follow-up: 28 months. 430 deaths were recorded (198 for Cabometyx® and 232 for everolimus).2 Median PFS and ORR data cut-off: May 2015. Median follow-up: 11.4 months for Cabometyx®, 11.5 months for everolimus.1
**All 658 randomly assigned patients were included in the analysis.
aRCC, advanced renal cell carcinoma; CI, confidence interval; HR, hazard ratio; IRC, independent review committee; OS, overall survival; PFS, progression-free survival; ORR, objective response rate; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
References
- Choueiri TK, et al. Lancet Oncol. 2016;17(7):917–927.
- Motzer RJ, et al. Br J Cancer. 2018;118(9):1176–1178.
For further information, please refer to the Prescribing Information or the Summary of Product Characteristics.
