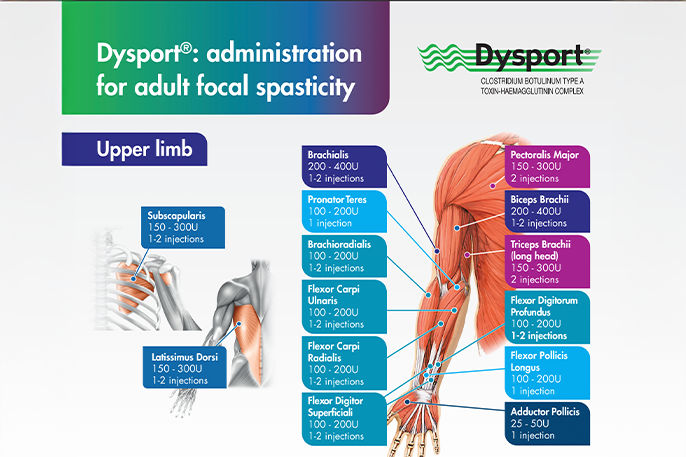
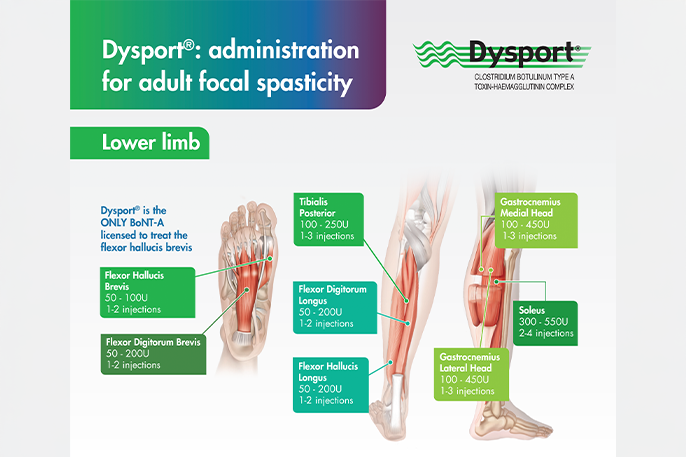
To enable long-lasting symptom control of adult spasticity, Dysport® should be injected at the recommended dose in the approved muscles. For every Dysport® injection, dosing should be tailored to the individual based on the size, number and location of muscles, severity of spasticity, any local muscle weakness, and the patient’s response to previous treatment and adverse event history.1

Dosing and administration
The dosing recommendations in the Dysport® SmPC should always be followed:1
| Upper limbs: doses greater than 1000 U and up to 1500 U can be administered when the shoulder muscles are also injected. The total dose recommended in the selected shoulder muscles is up to 500 U. |
| Lower limbs: maximum dose across lower limbs is 1500 U at a given treatment session. |
| No more than 500 U/1 ml should generally be administered at any single injection site. |
| Maximum combined dose across both upper and lower limbs is 1500 U at a given treatment session. |
Adult spasticity dilution tables2
Adult upper limb spasticity dilution table
Adult lower limb spasticity dilution table
| Volume Injected (ml) | Volume of injectable NaCl solution (0.9%) used to reconstitute a vial of Dysport® 500 U | ||
|---|---|---|---|
| 1ml | 2.5ml | 5ml | |
| Dysport® units delivered | |||
| 0.1 | 50 | 20 | 10 |
| 0.2 | 100 | 40 | 20 |
| 0.3 | 150 | 60 | 30 |
| 0.4 | 200 | 80 | 40 |
| 0.5 | 250 | 100 | 50 |
| 0.6 | 300 | 120 | 60 |
| 0.7 | 350 | 140 | 70 |
| 0.8 | 400 | 160 | 80 |
| 0.9 | 450 | 180 | 90 |
| 1.0 | 500 | 200 | 100 |
| 1.1 | 220 | 110 | |
| 1.2 | 240 | 120 | |
| 1.3 | 260 | 130 | |
| 1.4 | 280 | 140 | |
| 1.5 | 300 | 150 | |
| 1.6 | 320 | 160 | |
| 1.7 | 340 | 170 | |
| 1.8 | 360 | 180 | |
| 1.9 | 380 | 190 | |
| 2.0 | 400 | 200 | |
| 2.1 | 420 | 210 | |
| 2.2 | 440 | 220 | |
| 2.3 | 460 | 230 | |
| 2.4 | 480 | 240 | |
| 2.5 | 500 | 250 | |
| 2.6 | 260 | ||
| 2.7 | 270 | ||
| 2.8 | 280 | ||
| 2.9 | 290 | ||
| 3.0 | 300 | ||
| 3.1 | 310 | ||
| 3.2 | 320 | ||
| 3.3 | 330 | ||
| 3.4 | 340 | ||
| 3.5 | 350 | ||
| 3.6 | 360 | ||
| 3.7 | 370 | ||
| 3.8 | 380 | ||
| 3.9 | 390 | ||
| 4.0 | 400 | ||
| 4.1 | 410 | ||
| 4.2 | 420 | ||
| 4.3 | 430 | ||
| 4.4 | 440 | ||
| 4.5 | 450 | ||
| 4.6 | 460 | ||
| 4.7 | 470 | ||
| 4.8 | 480 | ||
| 4.9 | 490 | ||
| 5.0 | 500 | ||
| Volume Injected (ml) | Volume of injectable NaCl solution (0.9%) used to reconstitute a vial of Dysport® 500 U | ||
|---|---|---|---|
| 1ml | 2.5ml | 5ml | |
| Dysport® units delivered | |||
| 0.1 | 50 | 20 | 10 |
| 0.2 | 100 | 40 | 20 |
| 0.3 | 150 | 60 | 30 |
| 0.4 | 200 | 80 | 40 |
| 0.5 | 250 | 100 | 50 |
| 0.6 | 300 | 120 | 60 |
| 0.7 | 350 | 140 | 70 |
| 0.8 | 400 | 160 | 80 |
| 0.9 | 450 | 180 | 90 |
| 1.0 | 500 | 200 | 100 |
| 1.1 | 220 | 110 | |
| 1.2 | 240 | 120 | |
| 1.3 | 260 | 130 | |
| 1.4 | 280 | 140 | |
| 1.5 | 300 | 150 | |
| 1.6 | 320 | 160 | |
| 1.7 | 340 | 170 | |
| 1.8 | 360 | 180 | |
| 1.9 | 380 | 190 | |
| 2.0 | 400 | 200 | |
| 2.1 | 420 | 210 | |
| 2.2 | 440 | 220 | |
| 2.3 | 460 | 230 | |
| 2.4 | 480 | 240 | |
| 2.5 | 500 | 250 | |
| 2.6 | 260 | ||
| 2.7 | 270 | ||
| 2.8 | 280 | ||
| 2.9 | 290 | ||
| 3.0 | 300 | ||
| 3.1 | 310 | ||
| 3.2 | 320 | ||
| 3.3 | 330 | ||
| 3.4 | 340 | ||
| 3.5 | 350 | ||
| 3.6 | 360 | ||
| 3.7 | 370 | ||
| 3.8 | 380 | ||
| 3.9 | 390 | ||
| 4.0 | 400 | ||
| 4.1 | 410 | ||
| 4.2 | 420 | ||
| 4.3 | 430 | ||
| 4.4 | 440 | ||
| 4.5 | 450 | ||
| 4.6 | 460 | ||
| 4.7 | 470 | ||
| 4.8 | 480 | ||
| 4.9 | 490 | ||
| 5.0 | 500 | ||
Retreating: treatment may be repeated every 12–16 weeks based on clinical symptom return (or longer, as necessary, but not before 12 weeks).*1
Locating injection sites: although actual location of injection sites can be determined by palpation, use of injection guiding techniques, e.g. electromyography, electrical stimulation or ultrasound, is recommended to target injections.1
The recommended doses per treatment session for each upper limb and lower limb muscle group can be viewed in the administration guides below.
*The degree and pattern of muscle spasticity at the time of re-injection may necessitate alterations in the dose of Dysport® and muscles to be injected.1
References:
- Dysport® Summary of Product Characteristics.
- Dysport® dosing and administration guide.
Abbreviations:
SmPC, Summary of Product Characteristics; U, units.
For further information, please refer to the Prescribing Information or the Summary of Product Characteristics.