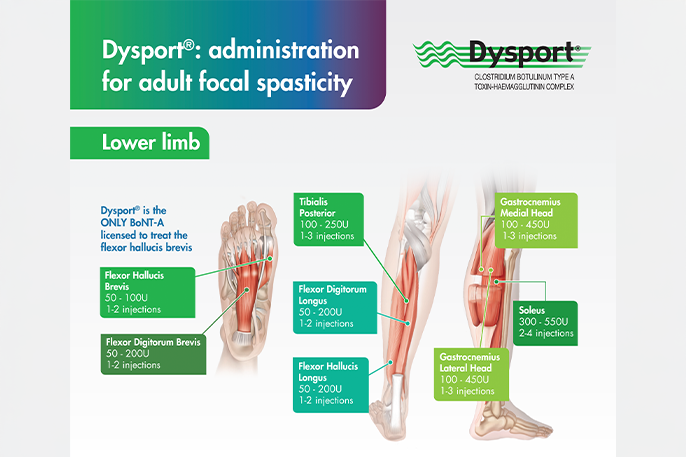
Dysport® administration guide for adult focal spasticity affecting the lower limbs
This leaflet provides the recommended Dysport® dosing and number of injection sites for lower limb muscle groups.
DYS-IE-000962 | August 2025
This website has been commissioned by Ipsen Pharmaceuticals Ltd. and is intended for Irish Healthcare Professionals. If you are not a Healthcare Professional, please click here.
Adverse event reporting information is available at the bottom of this webpage.
This website has been commissioned by Ipsen Pharmaceuticals Ltd. and is intended for an Irish audience
This leaflet provides the recommended Dysport® dosing and number of injection sites for lower limb muscle groups.
DYS-IE-000962 | August 2025


Adverse events should be reported.
Reporting forms and information can be found at www.hpra.ie or e-mail medsafety@hpra.ie.
The HPRA can also be contacted on +353 16764971. Adverse events should also be reported to Ipsen via email at pharmacovigilance.uk-ie@ipsen.com or phone on +353 1 8098256.
Reporting of side effects:
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly to the HPRA. Reporting forms and information can be found at www.hpra.ie or email medsafety@hpra.ie. Adverse events should also be reported to Ipsen via email at pharmacovigilance.uk-ie@ipsen.com or phone on +353 1 8098256. By reporting side effects, you can help provide more information on the safety of this medicine.