Dysport® demonstrates long-lasting symptom control between injections: significant reductions in muscle tone and increased range of motion, with time to retreatment of 12–16* weeks or longer.1–6

Efficacy information
More than a third of patients (36.9%) did not require retreatment until Week 16 or later after their previous Dysport® injection in a pivotal trial.4
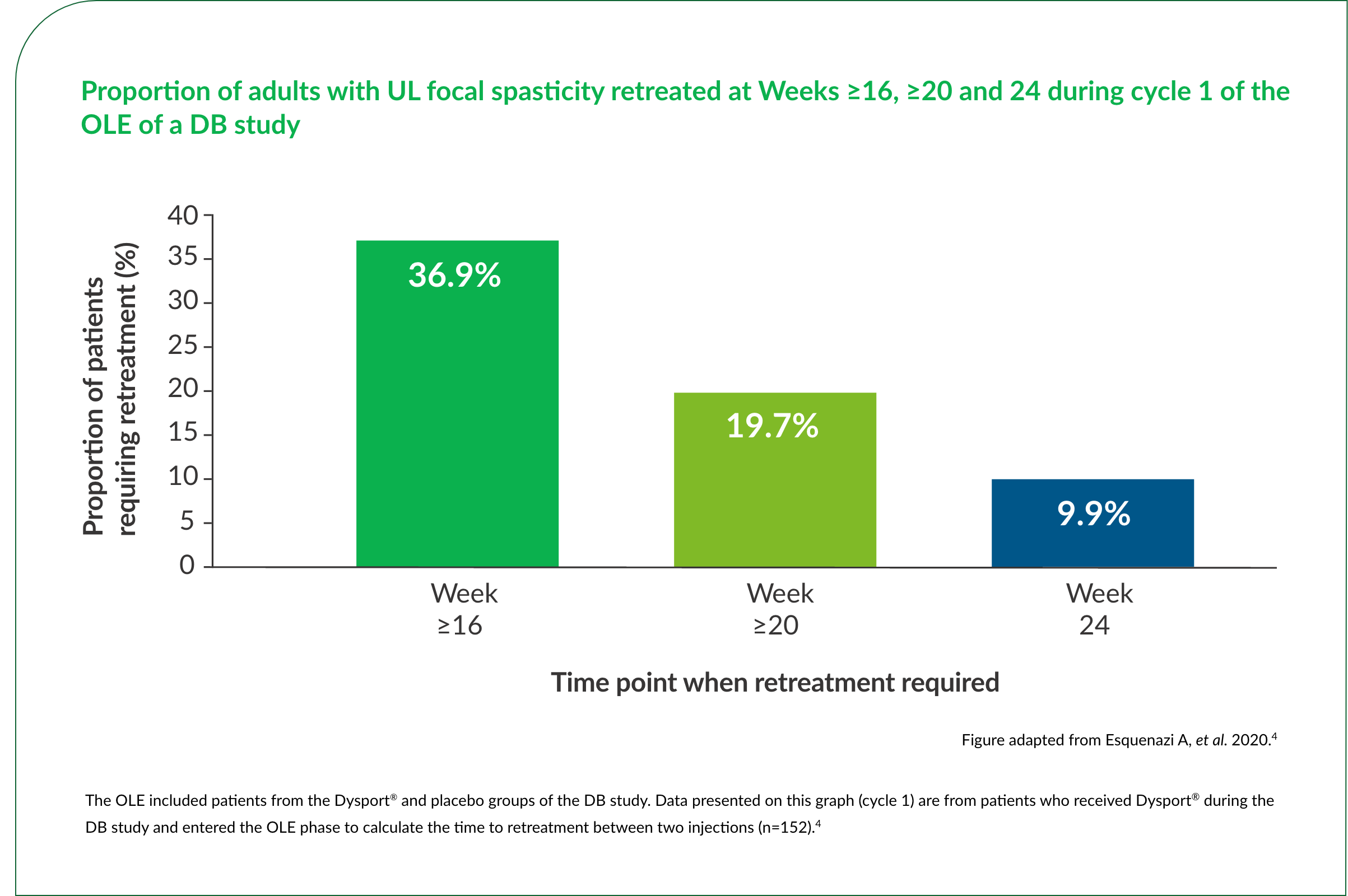
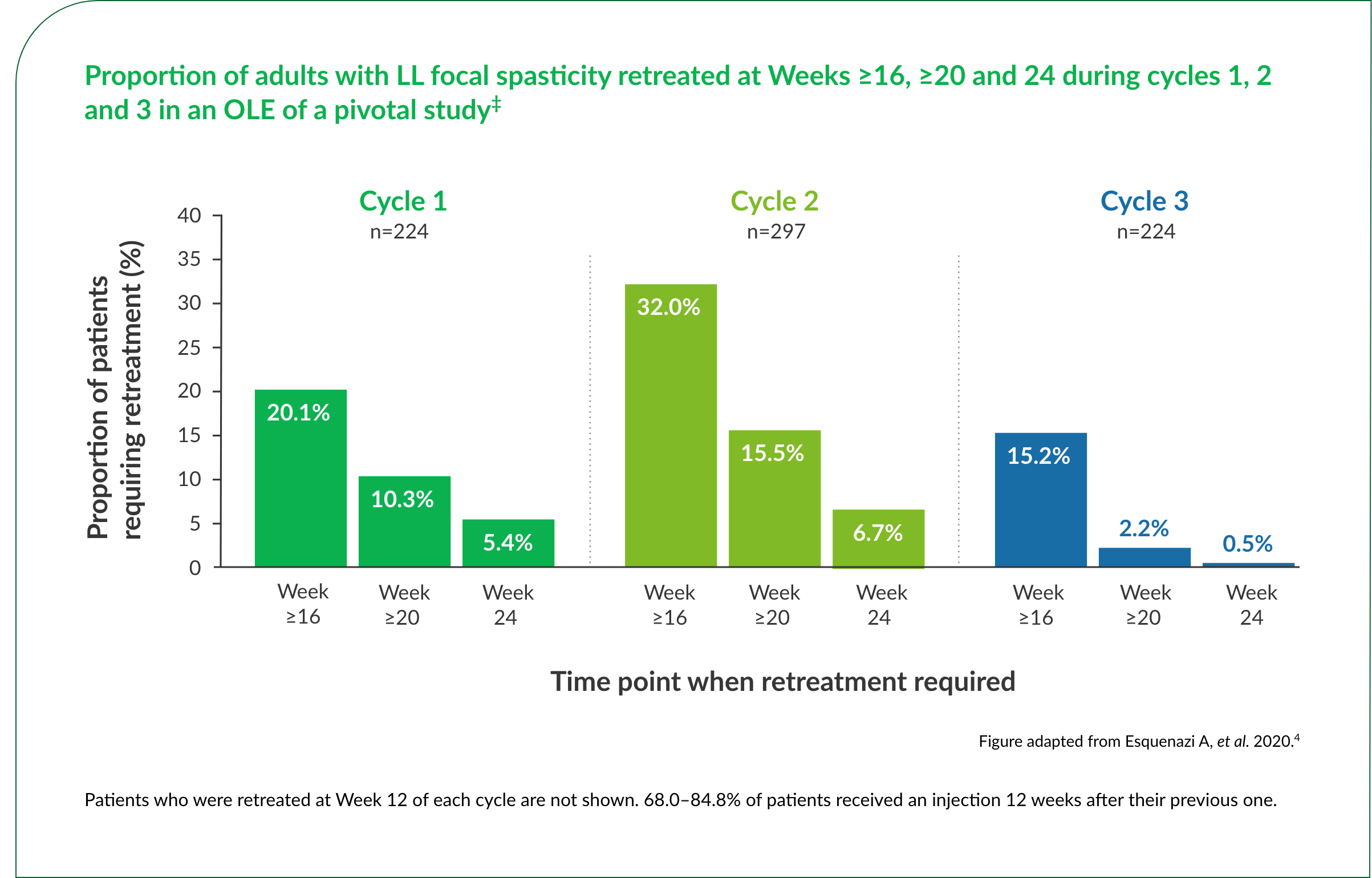
Up to 32%† of patients did not require retreatment until Week 16 or later over repeat Dysport® treatment cycles.3,4
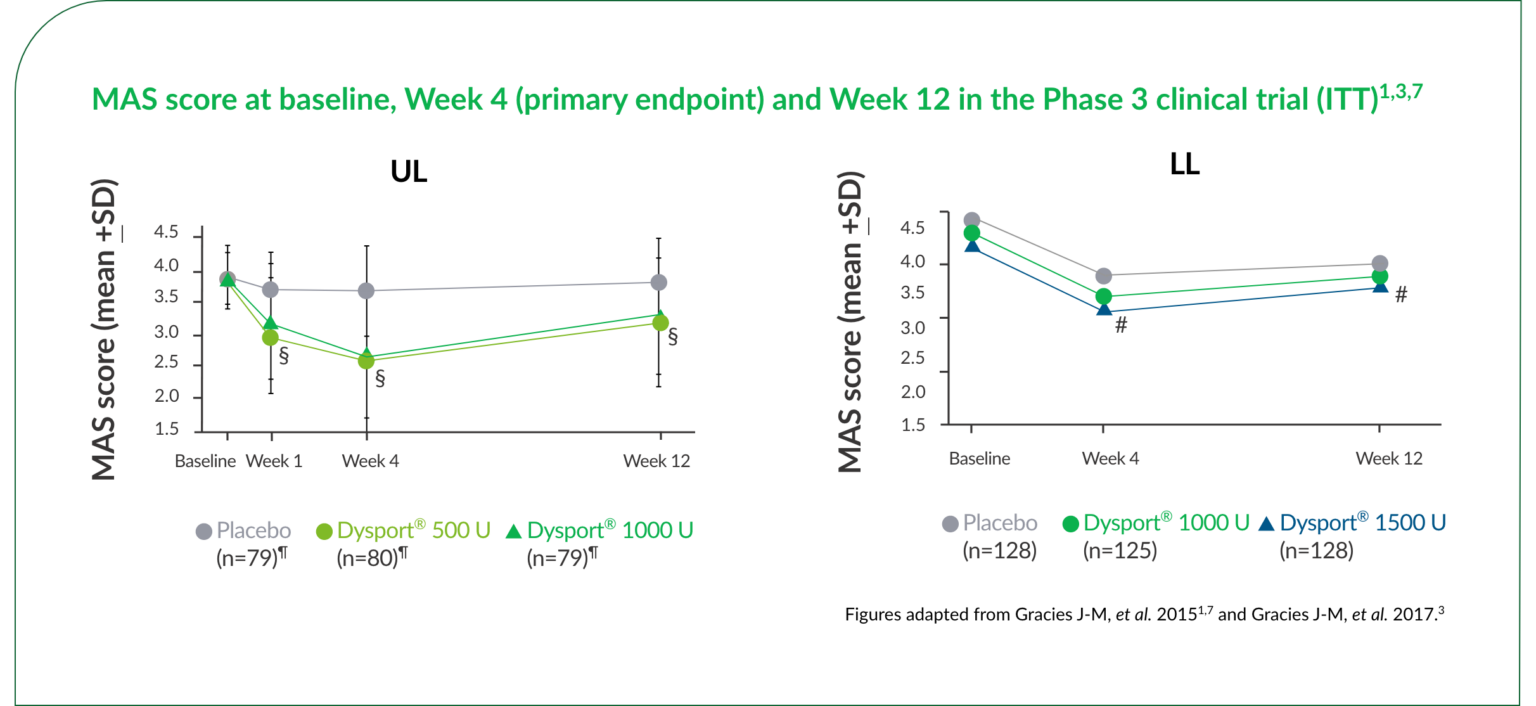
With Dysport®, significant reductions in muscle tone were sustained through Week 12.1,3,7
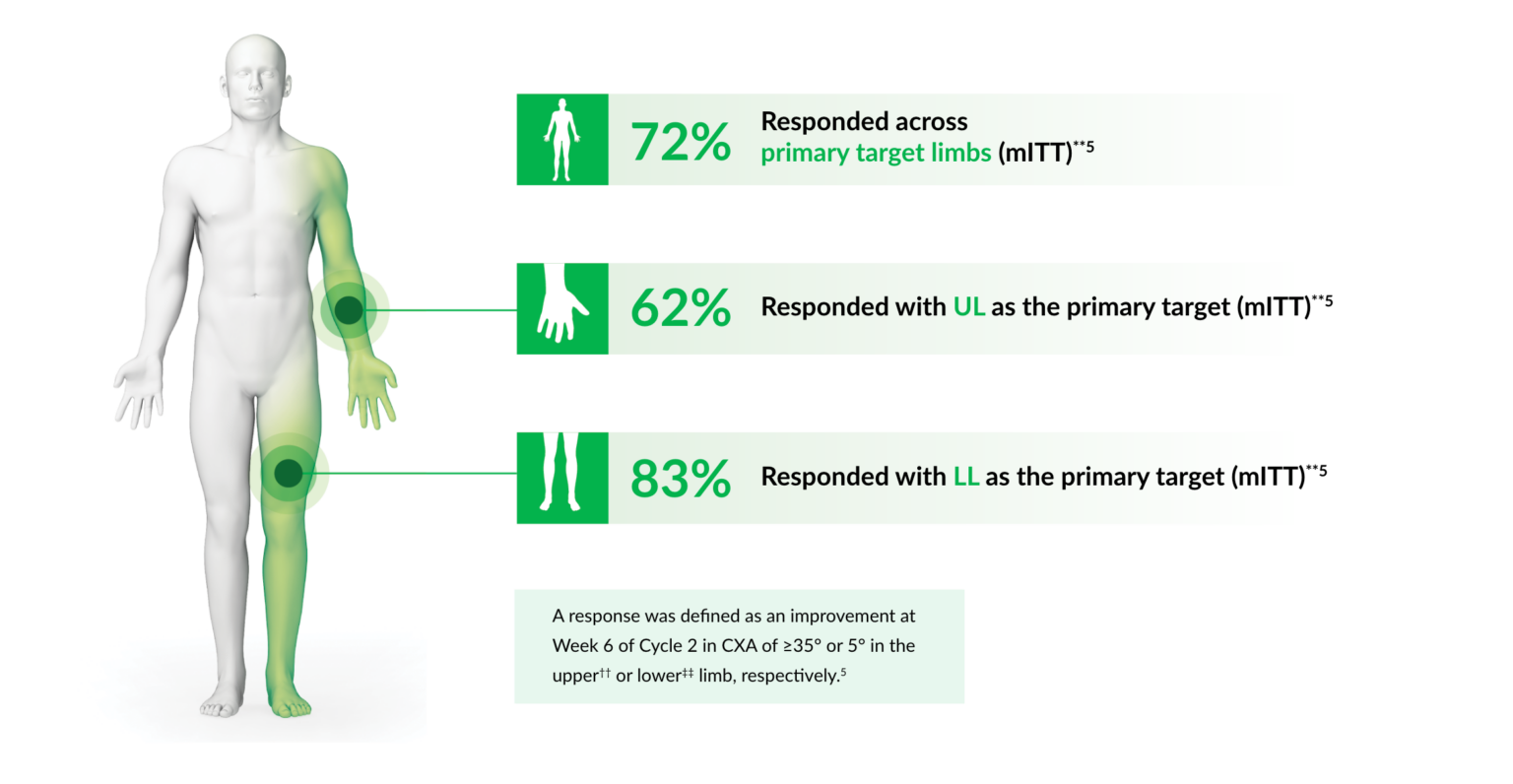
Dysport® can provide an increased range of motion, with most patients responding to treatment5
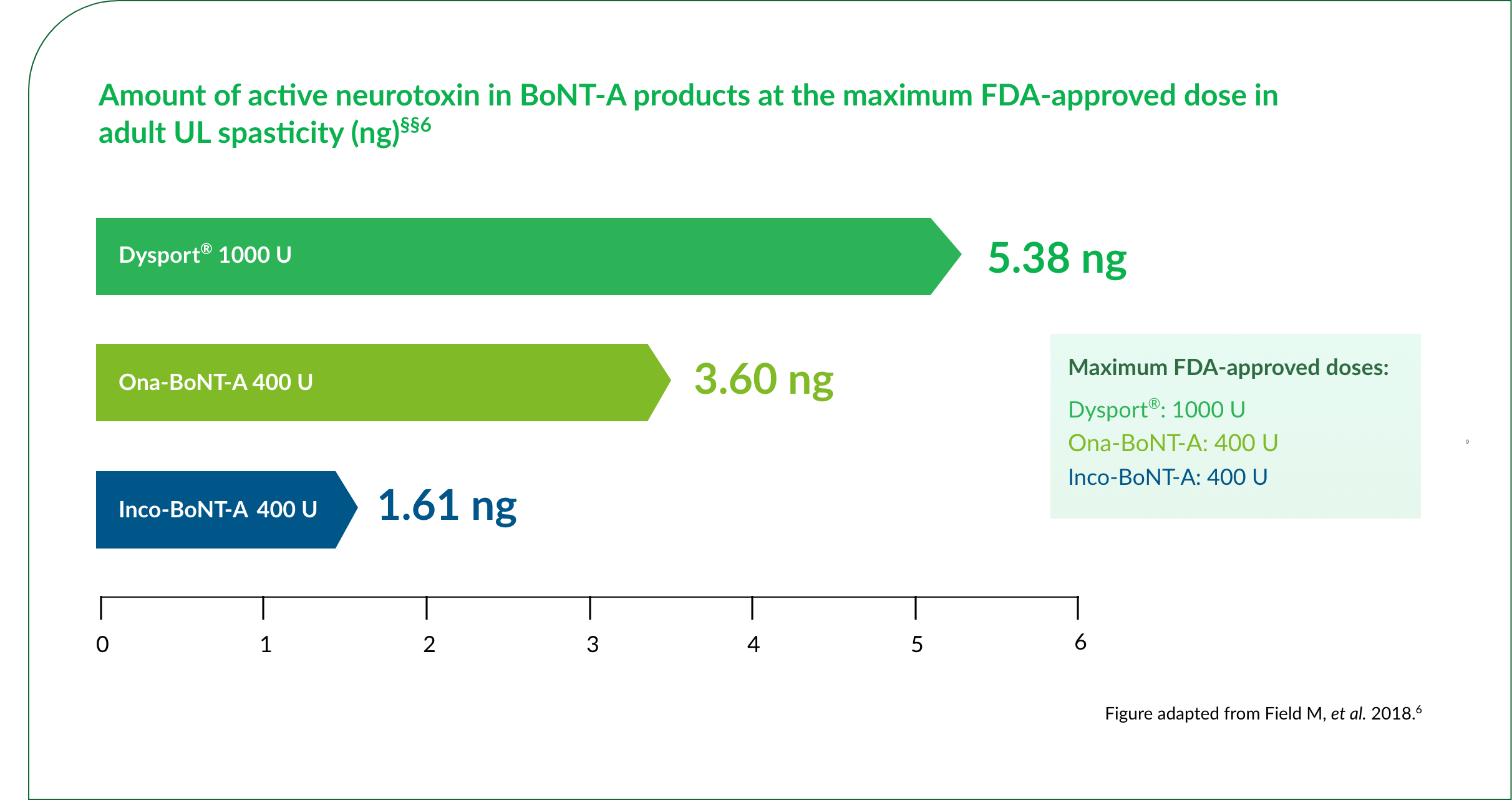
At the maximum approved dose of 1000 U, Dysport® contains 5.38 ng of active neurotoxin6
*Dysport® should not be administered more frequently than every 12 weeks.
†15.2–32%.3,4
‡Cycle 1 (n=224) included patients who were treated with Dysport® in the DB phase and entered the OLE phase. Cycles 2 (n=297) and 3 (n=224) included patients who received Dysport® in the OLE, including those who received placebo in the DB phase.4
§P<0.0001 vs placebo for both doses.1,7
¶Numbers at baseline.1,7
#P<0.05 vs placebo for 1500 U dose.3
**The mITT population included ITT patients with primary endpoint data at Week 6 of cycle 2.5 The ITT population included all patients with a defined primary treatment target limb who received ≥1 Dysport® injections and ≥1 days of guided self-rehabilitation contract therapy.5
††UL CXA defined as the sum of XA values against the resistance of elbow flexors, wrist flexors and extrinsic finger flexors.5
‡‡LL CXA defined as the sum of XA values against the resistance of soleus and gastrocnemius muscles.5
§§Amount of active neurotoxin at the maximum FDA-approved dose, according to a study that assessed the quantity and light chain activity of BoNT-A products, whereby 150 kDA BoNT-A was quantified by sandwich ELISA and light chain activity was assessed by the EndoPep assay. In both assays, results were assessed for the products against recombinant 150 kDa BoNT-A.6
References:
- Gracies J-M, et al. Lancet Neurol. 2015;14(10):992–1001.
- Gracies J-M, et al. Muscle Nerve. 2018;57(2):245–254.
- Gracies J-M, et al. Neurology. 2017;89(22):2245–2253.
- Esquenazi A, et al. Front Neurol. 2020;11:576117.
- Gracies J-M, et al. J Neurol Phys Ther. 2021;45(3):203–213.
- Field M, et al. Toxins. 2018;10(12):535.
- Gracies J-M, et al. Lancet Neurol. 2015;14(10):992–1001. (Table S3; supplementary material).
Abbreviations:
BoNT-A, botulinum toxin type A; CXA, composite range of active motion; DB, double-blind; ELISA, enzyme-linked immunosorbent assay; FDA, US Food and Drug Administration; Inco-BoNT-A, incobotulinumtoxinA; ITT, intention-to-treat; LL, lower limb; MAS, Modified Ashworth Scale; mITT, modified intention-to-treat; OLE, open-label extension; Ona-BoNT-A, onabotulinumtoxinA; U, units; UL, upper limb; XA, active range of motion.
For further information, please refer to the Prescribing Information or the Summary of Product Characteristics.

