
Efficacy information
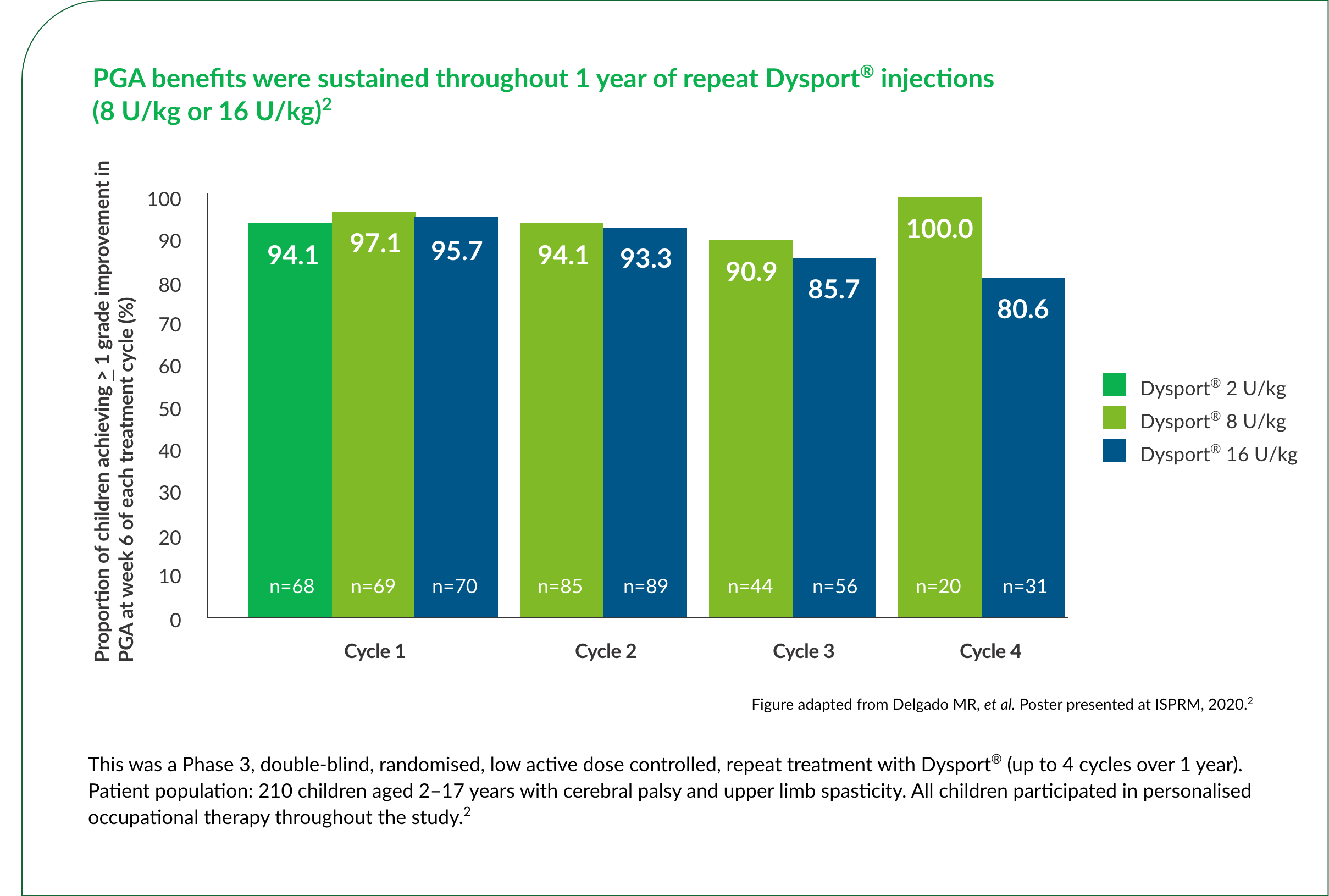
The majority of children with upper limb spasticity receiving Dysport® achieved a ≥1 grade improvement in PGA at Week 6 of the first treatment cycle.2
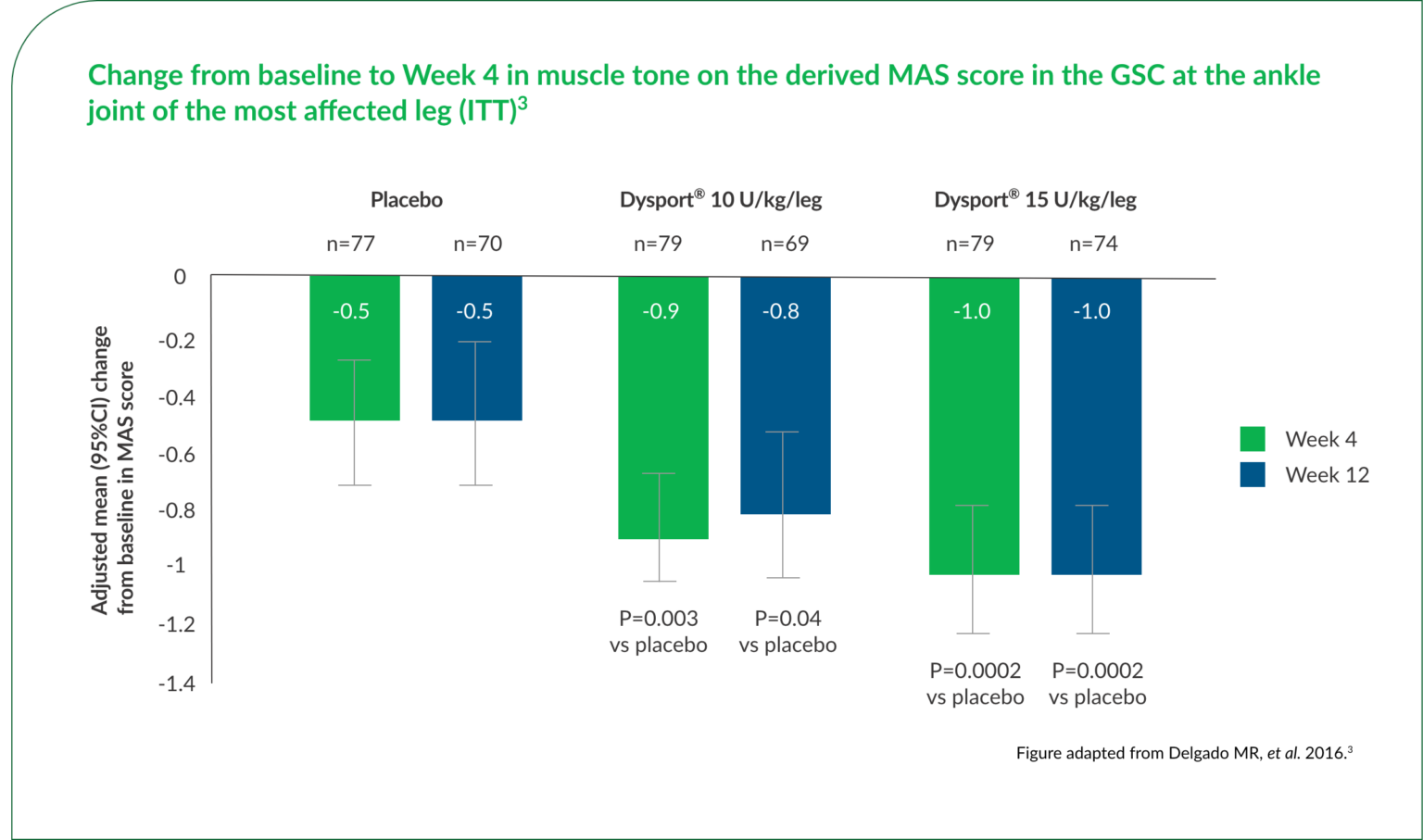
Dysport® significantly reduced muscle tone in the GSC at the ankle joint compared with baseline.3
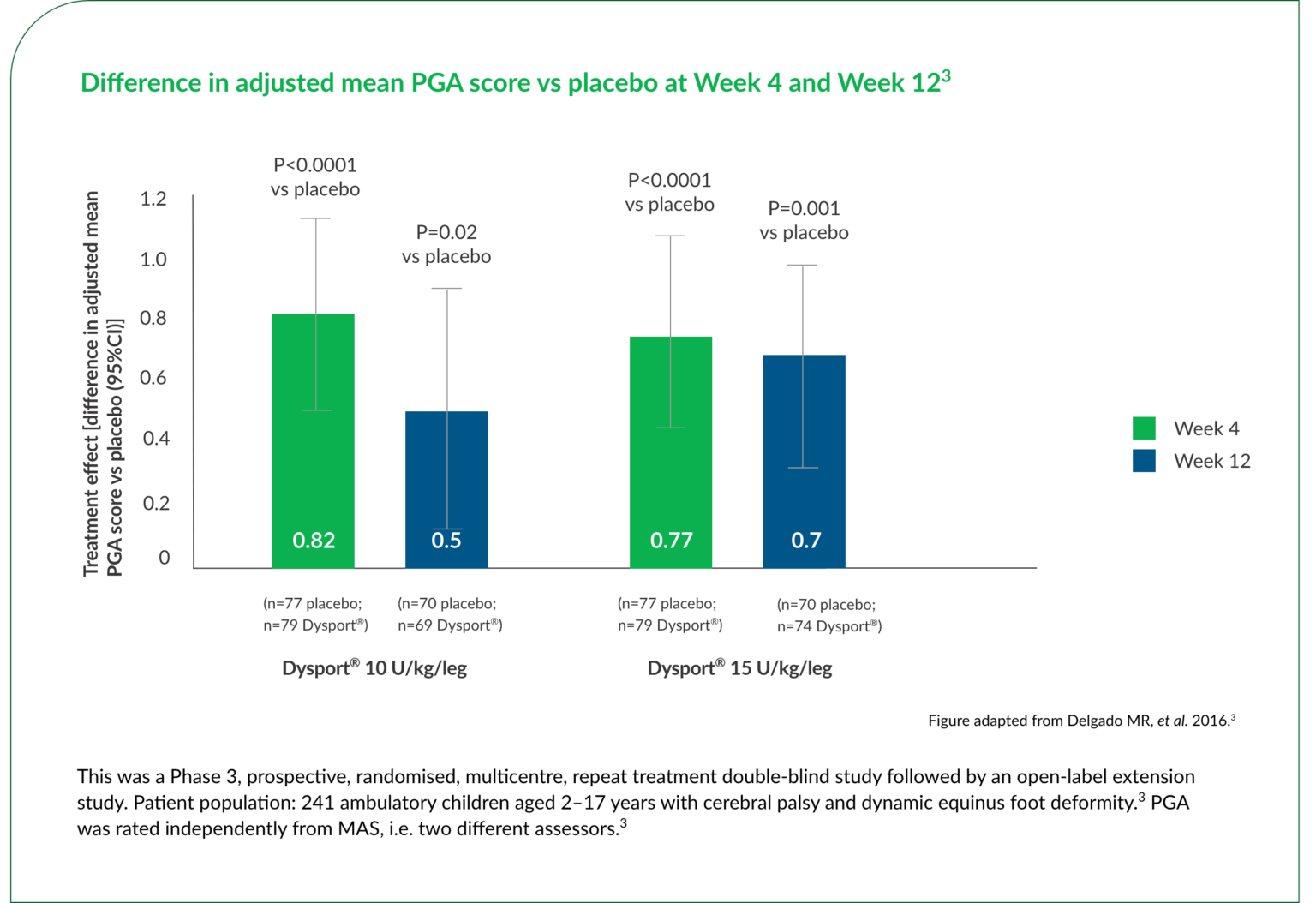
Dysport® significantly improved PGA compared with placebo in children with lower limb spasticity.3
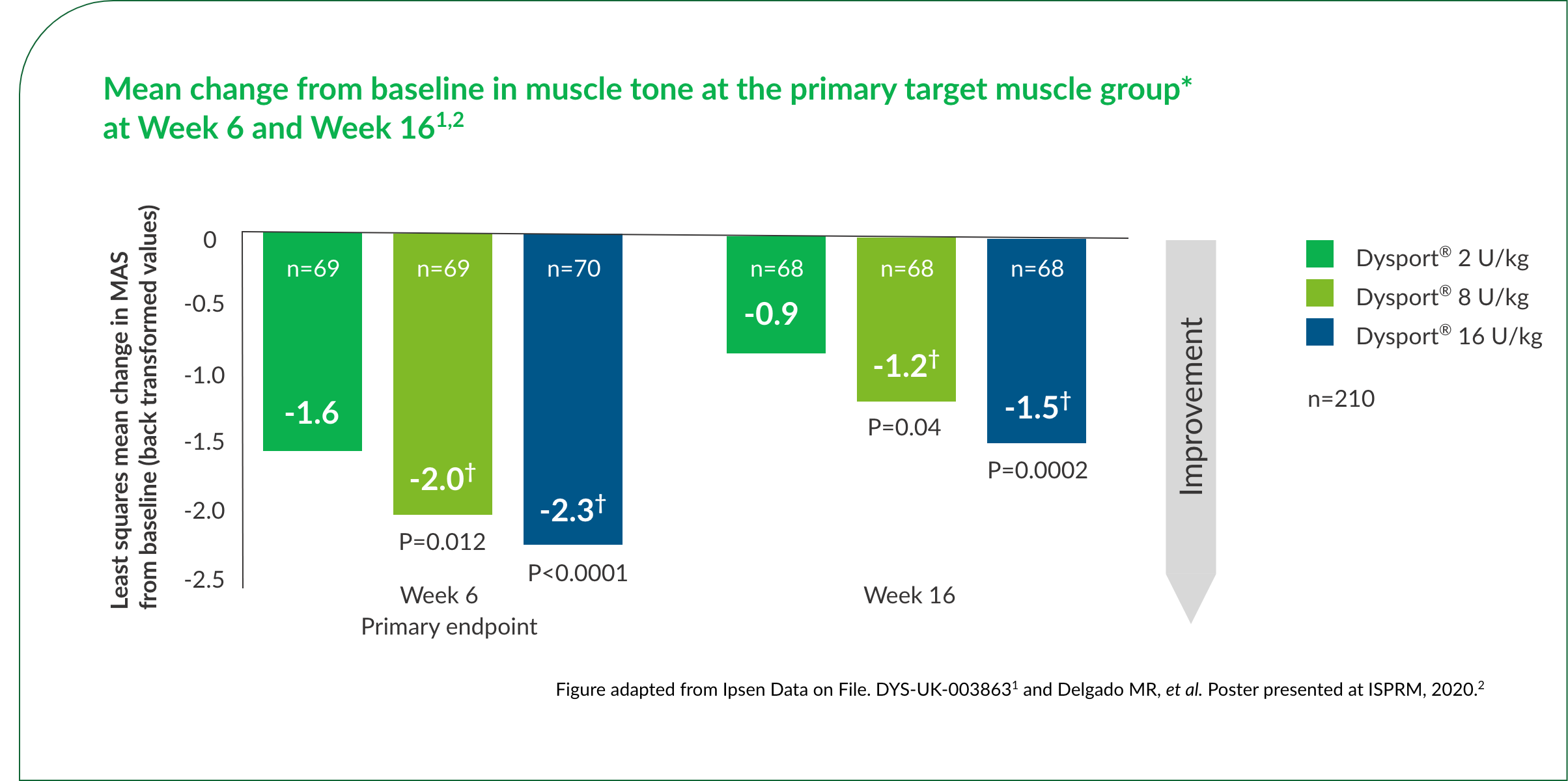
*Investigators chose either elbow or wrist flexors for the primary target muscle group.1,2
†Statistically significant vs 2 U/kg control group.1,2
References:
- Ipsen Data on File: DYS-UK-003863.
- Delgado MR, et al. Poster presentedat ISPRM. 2020.
- Delgado MR, et al. Paediatrics. 2016;137(2):e20152830.
Abbreviations:
GSC, gastrocsoleus muscle complex; ITT, intention-to-treat; MAS, Modified Ashworth Scale; PGA, Physician’s Global Assessment; U, units.
For further information, please refer to the Prescribing Information or the Summary of Product Characteristics.

