Reasons to choose Decapeptyl® (triptorelin)
There are more than 25 years of experience using Decapeptyl® for prostate cancer treatment in Ireland.1
This website has been commissioned by Ipsen Pharmaceuticals Ltd. and is intended for an Irish audience. If you are not a Healthcare Professional, please click here.
Adverse event reporting information is available at the bottom of this webpage.
This website has been commissioned by Ipsen Pharmaceuticals Ltd. and is intended for an Irish audience
There are more than 25 years of experience using Decapeptyl® for prostate cancer treatment in Ireland.1
Decapeptyl® clinical studies have shown that it can produce durable results that maintain castrate testosterone levels in patients with locally advanced and metastatic prostate cancer.2,3
With the 1- and 3-month dosing formulations, Decapeptyl® has improved lower urinary tract symptoms (LUTS) and the associated quality of life versus baseline.4
Prostate volume has also been reduced to a similar extent with Decapeptyl® versus goserelin and the 1-monthly strength leuprorelin.5,6


Decapeptyl® side effects are consistent across all three formulations.1,7,8
Very common (≥1 in 10) Decapeptyl® side effects in men have included:1,7,8
Read more about Decapeptyl® side effects
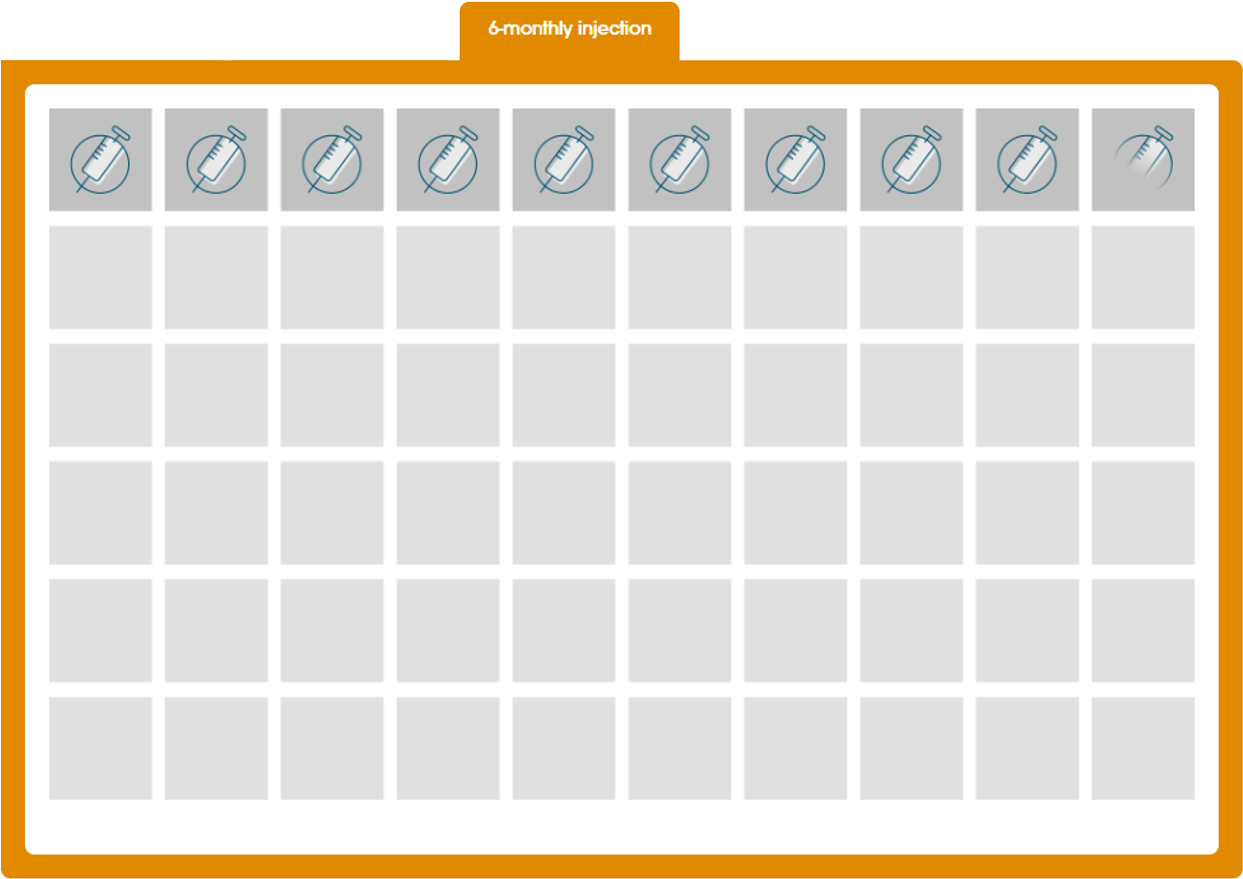
Decapeptyl® gives you the flexibility of offering your prostate cancer patients 6-, 3-, and 1-monthly dosing.1,7,8
The 6-monthly formulation is designed to deliver 22.5 mg of triptorelin over a 6-month period after a single intramuscular injection.8

Prostate cancer patients on 1- and 3-monthly LHRHa have perceived that 6-monthly LHRHa therapy could have benefits, such as:9
Adapted from Shulman C, 2007.9
Data are from face-to-face interviews (n=200) in France, Germany, Italy, the Netherlands and Spain.9
The study was conducted in men diagnosed with prostate cancer who were receiving LHRHa treatment.9 The survey was carried out before the 6-monthly Decapeptyl® was available. The majority (79%) of respondents received hormone injections once every 3 months, and a fifth (21%) received injections once every month.9
The aim was to: understand the attitudes of patients with prostate cancer toward the disease in general and to the use of hormone therapy as a treatment; assess unmet needs in the management of prostate cancer; gauge patient receptivity to a potential 6-monthly formulation of LHRHa.9

The availability of 6-monthly Decapeptyl® dosing means fewer healthcare-patient interactions for injections versus 1- and 3-monthly dosing, potentially freeing up time for discussing other patient concerns.10
Using Decapeptyl® 6-monthly potentially saves resources and time versus the 1- and 3-monthly LHRHa injection frequencies, as demonstrated by peer-reviewed, real-world, retrospective, multicentre data from the UK-based Decapeptyl SERvice Evaluation (DESERVE) study.*†10
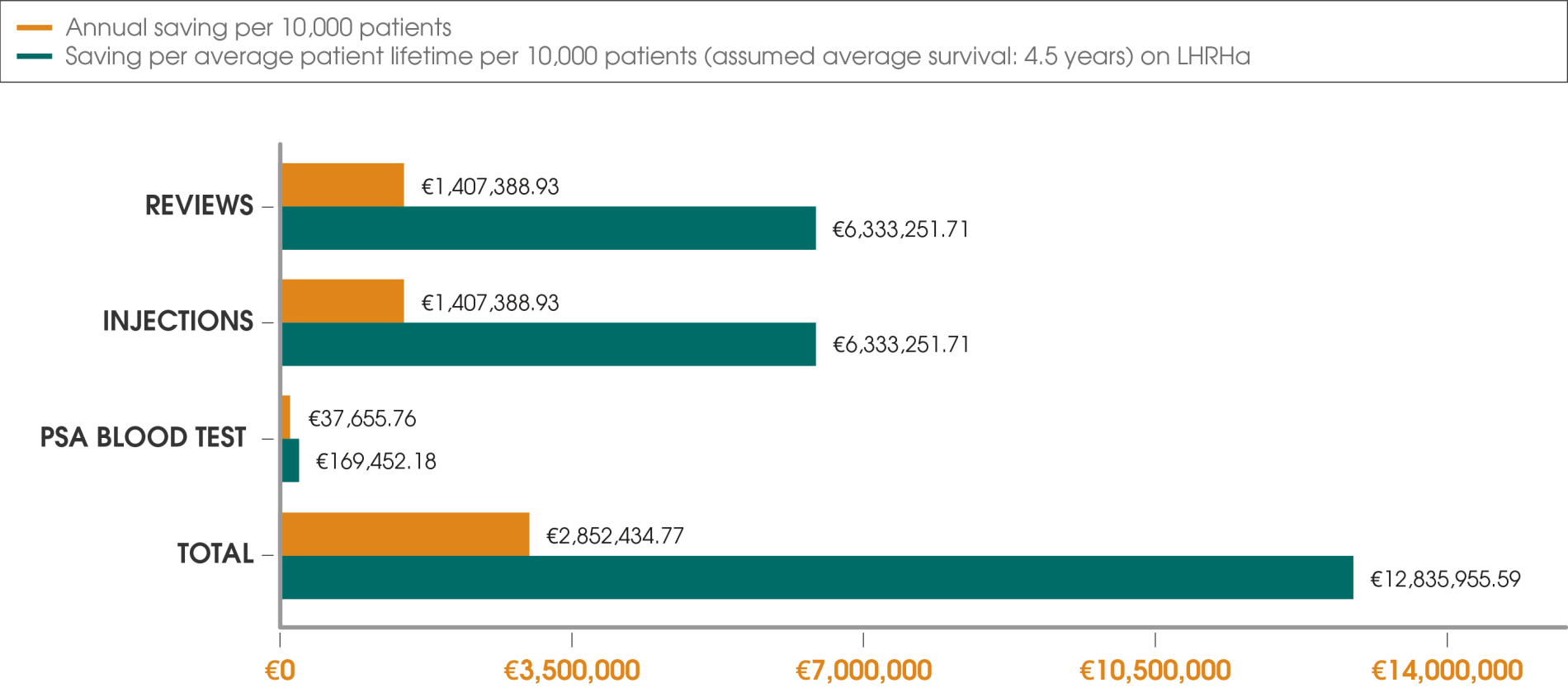
Adapted from Cornford P et al., 2018.10
Cost assumptions were correct as of December 2015.
*Cost reduction was based upon the reduction in the total number of patient–healthcare interactions (41.5%; p<0.0001).
†Cost reduction estimates were reported in pound sterling by Cornford P et al., 2018 and converted to the euro for use in this figure. The currency conversion calculations were based on the closing exchange rate of 1 GBP = 1.1449 EUR,11 reported on the publication date of the DESERVE study (5th November 2018).
Switching to Decapeptyl® 6-monthly from the 1- and 3-monthly LHRHa injection frequencies has the potential to save resources, as demonstrated by data from the Decapeptyl SERvice Evaluation (DESERVE) study.10
All reductions in resources were significant (p<0.0001).
*Control of PSA was maintained in patients who were switched to Decapeptyl® 6-monthly from the 1- and 3-monthly LHRHa injection frequencies.11 Read more about PSA control in the DESERVE study.
See the reduction in injection frequency that can be achieved with Decapeptyl® 6-monthly.
The graphical representations shown are based on the following assumptions:11


A UK-based study showed that switching to Decapeptyl® 6-monthly from the 1- and 3-monthly LHRHa injection frequencies may save:*†10
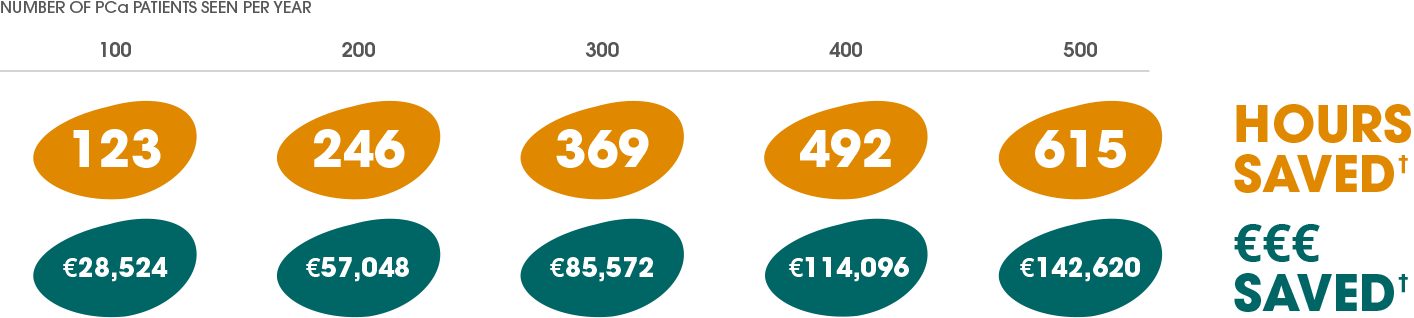
*Patient-healthcare interaction costs were correct at the time of analysis. The costs do not contain other resource use costs and may vary between primary and secondary care.
†Hours saved calculations: 73.8 mins x 100 patients / 60 mins = 123 hours; 73.8 mins x 200 patients / 60 mins = 246 hours; 73.8 mins x 300 patients / 60 mins = 369 hours; 73.8 mins x 400 patients / 60 mins = 492 hours; 73.8 mins x 500 patients / 60 mins = 615 hours. €€€ saved calculations: £249.14 x 100 patients = £24,914; £249.14 x 200 patients = £49828; £249.14 x 300 patients = £74,742; £249.14 x 400 patients = £99,656; £249.14 x 500 patients = £124,570. The cost saving of £249.14 per patient per year was reported in the DESERVE study. The output of cost saved calculations reported in this figure was converted from pound sterling to euro. The currency conversion calculations were based on the closing exchange rate of 1 GBP = 1.1449 EUR,11 reported on the publication date of the DESERVE study (5th November 2018).
Adverse events should be reported.
Reporting forms and information can be found at www.hpra.ie or e-mail medsafety@hpra.ie.
The HPRA can also be contacted on +353 16764971. Adverse events should also be reported to Ipsen via email at pharmacovigilance.uk-ie@ipsen.com or phone on +353 1 8098256.
Reporting of side effects:
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly to the HPRA. Reporting forms and information can be found at www.hpra.ie or email medsafety@hpra.ie. Adverse events should also be reported to Ipsen via email at pharmacovigilance.uk-ie@ipsen.com or phone on +353 1 8098256. By reporting side effects, you can help provide more information on the safety of this medicine.